5 things to know about the new RSV vaccine
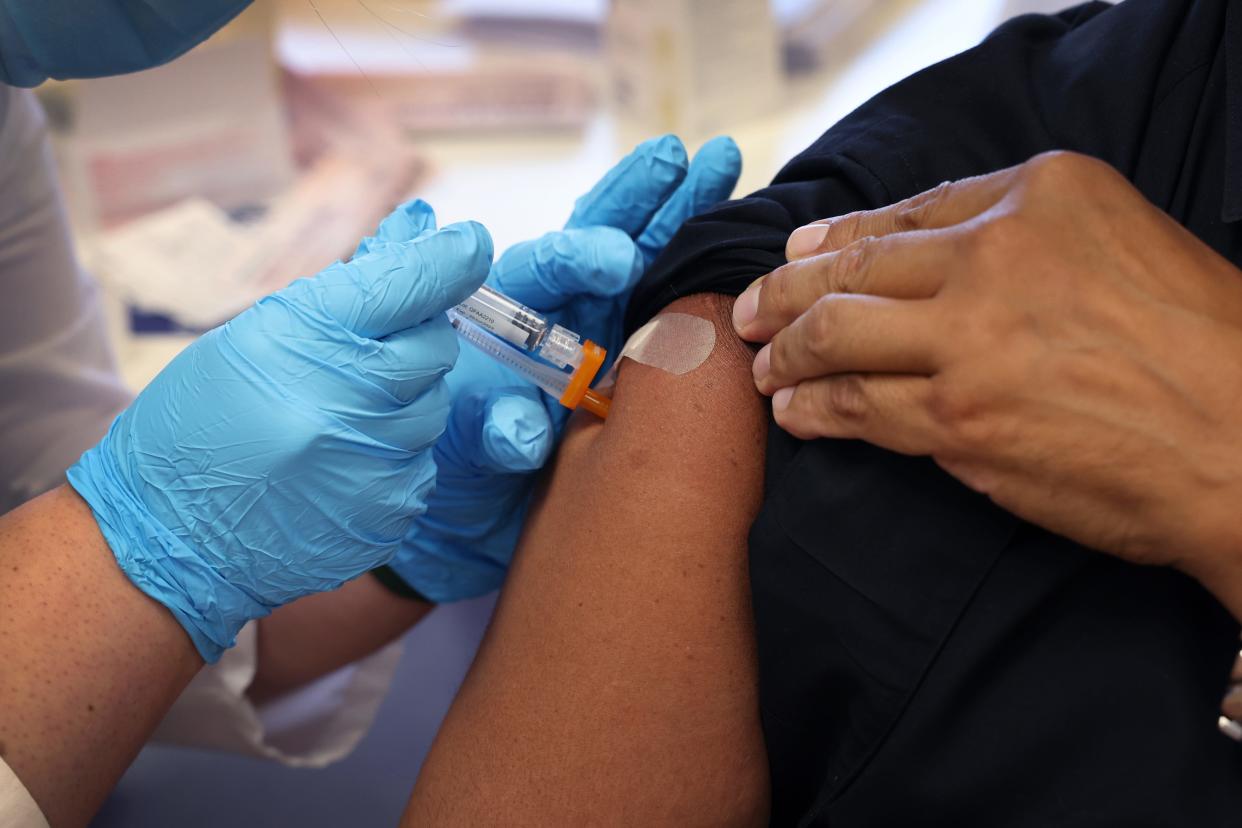
The U.S. Food and Drug administration this month approved a new vaccine to prevent respiratory syncytial virus (RSV) for ages 60 and older.
RSV is a highly contagious airborne virus that infects the lungs and sometimes causes other respiratory illnesses such as pneumonia and bronchiolitis, which can be life-threatening in older adults.
The Centers for Disease Control (CDC) estimates that between 60,000-160,000 older adults are hospitalized every year due to RSV, and between 6,000 to 14,000 deaths are attributed to RSV in adults 65 and older.
Approval of the vaccine, Arexvy, came after data was collected during an ongoing three-year clinical study of about 25,000 adults 60 years and older in the U.S. and internationally.
RSV season typically runs from about December through February, ramping up in the fall and decreasing in the early spring.
The vaccine is a breakthrough people have been waiting some 60 years for, said Dr. Katie Sharff, chief of infectious disease for Kaiser Permanente Northwest.
“There’s definitely a need to have an additional layer of protection against RSV,” Sharff said.
Who can get the vaccine and when?
The vaccine only will be available for those 60 years of age and older.
Individuals wanting to get an RSV vaccine can expect to do so sometime in the fall. It will not be available until then because a CDC committee is providing recommendations for best medical practices in distribution and use.
Will more than one vaccination be needed?
Currently, Arexvy is a single dose vaccine.
As the 3-year clinical study progresses and efficacy of the vaccine continues to be studied, another dose could be recommended, Sharff said
How effective and safe is the vaccine?
In the first year of the main trial, Arexvy was 83% effective in reducing the risk of lower respiratory tract disease caused by RSV and 94% effective in preventing severe illness.
The most commonly reported side effects by study participants were injection site pain, fatigue, muscle pain, headache and joint stiffness and pain.
Among the some 25,000 clinical trial participants,10 who received the vaccine and four who received a placebo reported atrial fibrillation, or irregular rapid heart rhythm, within 30 days.
The vaccination has been approved as safe for use by the Food and Drug Administration, and the risk for side effects, such as atrial fibrillation, will continue to be reviewed in a post-marketing study.
Can you get other vaccinations at the same time?
It is not known whether the RSV vaccine can be co-administered with vaccinations for COVID-19 and influenza.
The CDC will review this in the coming months, and medical recommendations for co-administration of Arexvy will be provided prior to the rollout of the vaccine, said Sharff.
What about children and infants?
In infants and children, contraction of RSV also can be life-threatening, especially in preterm babies, those under six months old, and children with weakened immune systems, neuromuscular disorders, and chronic lung disease, according to the Center for Disease Control and Prevention.
RSV is the cause of death in the U.S. every year for up to 300 children under five.
Pfizer has developed a vaccine for women between 24 and 36 weeks of pregnancy that is intended to prevent RSV in infants. This vaccine has not yet been approved by the Food and Drug Administration, so is not yet available to the public.
In a clinical trial of the vaccine with nearly 7,400 participants, it lowered the risk of severe RSV disease in infants by 82% within three months after birth. By around six months, efficacy was around 69%.
Sydney Wyatt covers healthcare inequities in the Mid-Willamette Valley for the Statesman Journal. Send comments, questions, and tips to her at SWyatt@gannett.com, (503) 399-6613, or on Twitter @sydney_elise44
The Statesman Journal’s coverage of healthcare inequities is funded in part by the M.J. Murdock Charitable Trust, which seeks to strengthen the cultural, social, educational, and spiritual base of the Pacific Northwest through capacity-building investments in the nonprofit sector.
This article originally appeared on Salem Statesman Journal: RSV vaccine available for older adults

