The 7 Diatomic Elements That Can't Stand to Be Alone
Diatomic elements hate to be alone — so much so that they just aren't found as single atoms.
Instead, they're always two atoms of the same pure element bonded together. It's right in the name: Di- means "two," and atomic means "of the atoms." And elements are the basic building blocks of the universe.
What Are the 7 Diatomic Elements?
On the periodic table, which contains 118 elements, there are only seven diatomic elements:
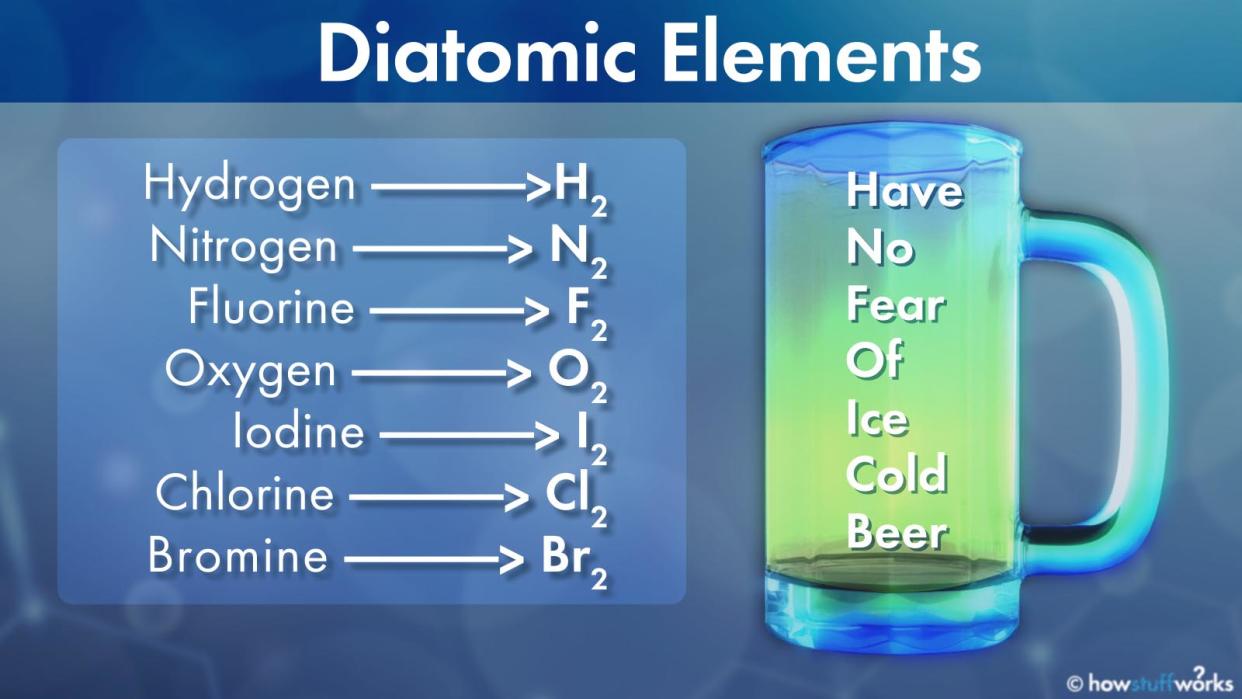
Hydrogen: H2
Nitrogen: N2
Fluorine: F2
Oxygen: O2
Iodine: I2
Chlorine: Cl2
Bromine: Br2
Except for hydrogen, which appears on the top left-hand side of the periodic table, the diatomic elements touch, forming an upside-down L shape. So if you spot one, you should be able to easily find all of them.
How to Remember the 7 Diatomic Elements
Need an easy way to remember these seven? Try this mnemonic: Have No Fear Of Ice Cold Beer. The first letter of each word will remind you of each diatomic element.
Diatomic Elements Are Everywhere
There are only seven diatomic elements that default to this double bond. Five of them — hydrogen, nitrogen, fluorine, oxygen and chlorine — are gases at room temperature and normal pressure. They also go by the name "elemental gases."
Bromine is always a liquid, while iodine can be a liquid or solid when at room temperature, depending on several factors. All seven are nonmetallic.
Diatomic elements are not rare — on the contrary! Nitrogen and oxygen, in their diatomic forms N2 and O2, make up 99 percent of the atmosphere on Earth. That's the opposite of rare.
Diatomic Molecules
Other elements can bond with diatomic elements to form diatomic molecules. That's how we get table salt (sodium + chlorine = NaCl, sodium chloride).
You can find a diatomic molecule everywhere. Some other elements can form diatomic molecules, but the bonds are very weak and unstable. They don't stay diatomic for long. Only the seven diatomic elements form strong bonds and appear in this form almost always.
Now That's Elemental
Elements can also be monatomic, which means there's only one atom. (Mon- means "one.") Helium is a monatomic element. And oxygen can be triatomic, with the oxygen atoms forming a triple bond. That's what we commonly call "ozone."
Original article: The 7 Diatomic Elements That Can't Stand to Be Alone
Copyright © 2023 HowStuffWorks, a division of InfoSpace Holdings, LLC, a System1 Company

