Baker: New tools can change mosquitoes' DNA, but should it be done?
Editor's note: Part 1
Suppose Sauron — or perhaps Gandalf — were to offer you a magical golden ring with the power to rid the world of mosquitoes once and for all. And with their demise, to save countless human lives from the many diseases for which mosquitoes are the sole or primary vectors:
Malaria, dengue, West Nile virus, chikungunya, yellow fever, filariasis, tularemia, encephalitis, Zika fever, Keystone Virus, Rift Valley Fever…

And not just mosquitoes. From within the folds of his cloak the wizard draws out an array of equally luminous rings with the power to cure genetic disorders like cystic fibrosis, hemophilia, and Down’s syndrome, to rid cities of mice and rats, and free the world’s farms of weeds and insect pests without the use of pesticides.
Would you take them?
Those rings exist and are now being refined and tested in the Elvin forges of academic, commercial, and government research facilities around the world. They go by various names, but collectively may be referred to as CRISPR-Cas Genome Editing Systems.
It is not a matter of good vs. bad
Not nearly so romantic as 19 rings of power with “One Ring to rule them all, One Ring to find them, One Ring to bring them all and in the darkness bind them.”
And yet, not so black-and-white nor good vs. bad as the science fiction tales with which I spent so much of my youth. I don’t mean to clothe CRISPR with sinister misgivings, but all technologies with the potential to change the world — rings of power, if you will — deserve serious and informed consideration, and not only by scientists, ethicists and politicians.
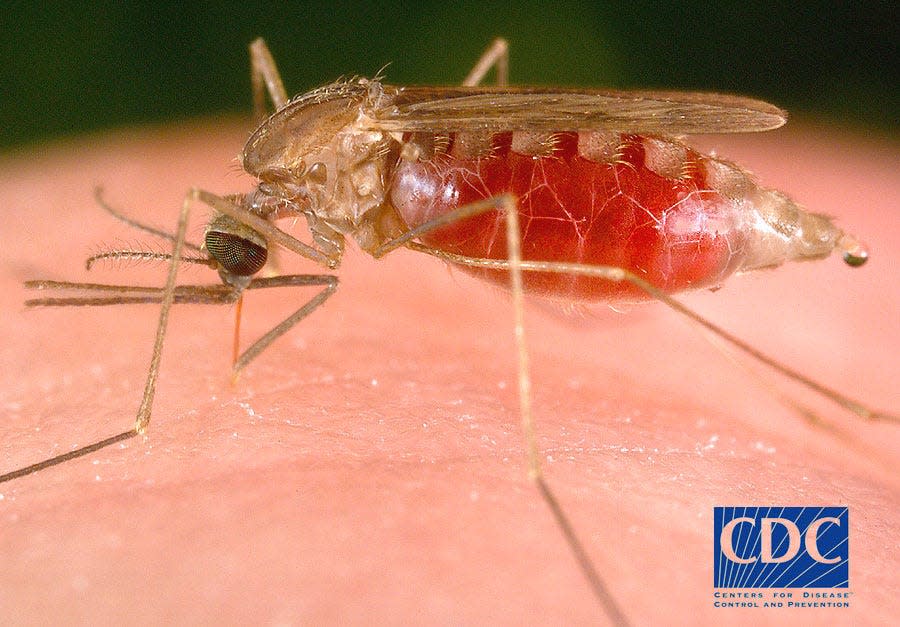
The World Health Organization’s data for 2020 estimated that malaria sickened close to 241 million people and killed some 670,000 worldwide, with 90% of those cases occurring in Africa where more than 80% of the deaths occurred among children under 5 years of age.
Who should make the final decisions to release modified mosquitoes?
Discussions are underway about possible experimental releases of CRISPR-modified Anopheles mosquitoes (the main carriers of malaria) in Burkina Faso, Mali, Ghana or Uganda in the near future, with the very real potential for eliminating the mosquitoes from those areas. But who will get to make the final decision to release these genetically modified animals into the wild?
So then, CRISPR-Cas Genome Editing Systems. This is complex stuff.
CRISPR — Clustered Regularly Interspaced Short Palindromic Repeats — refers to sections of DNA found in about 50% of bacterial genomes that have been analyzed and 90% of archaea. A given CRISPR includes bits of genetic material from viruses that had previously infected the bacterial or archaean cell. It’s like the cell’s virus memory bank, and it will help the cell defend itself against those viruses if they ever attack again.
“Cas” is shorthand for a group of CRISPER-associated proteins. These proteins contain one or more copies of the viral genetic material that’s been stored in a CRISPR. If the Cas protein, while roaming around the cell, bumps into a piece of free-floating viral genetic material that matches the bit it’s carrying, it means the cell has been re-invaded by that type of virus.
Science fiction is now science fact in the lab
The critical point here is that the Cas protein will then latch onto the invader’s DNA (or RNA), cut it, and thereby destroy it. That’s how Cas proteins defend the cell against viral invaders.
But researchers have learned how to commandeer the system for their own uses. By replacing the bit of viral genetic material held by a Cas protein with DNA from a “target gene” pulled from almost any type of animal, plant or fungus, scientists can make a Cas protein they can insert into a living organism of their choice, where it will find and cut that gene.
Genome-editing is the modification of an organism’s DNA at a specific site selected by a researcher who may choose to either delete some of the DNA at that site or tuck in a new piece of DNA. In doing so, she or he can modify what that DNA does within the organism.
Our story so far: Scientists now have the ability to make Cas proteins that can find and cut a piece of DNA at almost any desired location within almost any organism. Using genome editing tools (basically other types of proteins that have been modified to do the researchers’ bidding), the scientists can then add or delete a piece of DNA at that location.
One more item: In a 2018 laboratory study, scientists inserted a piece of DNA into the sex-determining gene of a few Anopheles mosquitoes that made females sterile. Even though it was clearly disadvantageous to the mosquitoes, the sterility-causing gene replaced all normal sex-determining genes within seven generations, causing the caged population to die out.
It’s an example of a “gene drive” which I’ll discuss in Part II of this account. But what it means, is that humanity now has a ring of power with the potential to rapidly and permanently alter the living world to its liking.But at what cost?
Ken Baker is a retired professor of biology and environmental studies. If you have a natural history topic you would like Dr. Baker to consider for an upcoming column, please email your idea to fre-newsdesk@gannett.com.
This article originally appeared on Fremont News-Messenger: Baker: Ability to alter mosquito DNA demands serious consideration

