Bottles of an ADHD drug got recalled. They might contain completely different meds
One lot of a drug used to treat ADHD and narcolepsy has been recalled because the medication in the bottle might not match the medication on the label.
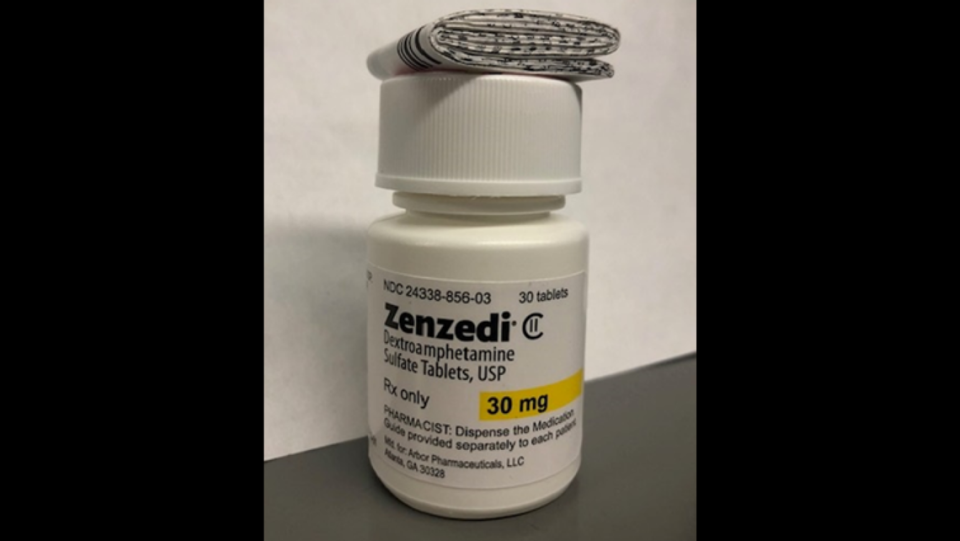
The label says what should be inside the bottles are 30 tablets of Zenzedi, 30 mg strength. But, the recall notice from Azurity Pharmaceuticals said, a Nebraska pharmacist opened a bottle to find an antihistamine, carbinoxamine maleate.
Taking the antihistamine instead of Zenzedi could lead to “functional impairment and an increased risk of accidents or injury,” the recall notice’s risk statement says. “Patients who unknowingly consume carbinoxamine could experience adverse events which include, but are not limited to, drowsiness, sleepiness, central nervous system depression, increased eye pressure, enlarged prostate urinary obstruction, and thyroid disorder.”
For people with narcolepsy or attention deficit hyperactivity disorder, “there is a reasonable probability that accidents or injuries that occur due to the sedating effects of carbinoxamine could lead to ongoing disability or death in severe cases, particularly if individuals who use it engage in activities requiring significant focus and alertness (e.g., driving, operating heavy machinery).”
READ MORE: Check your medicine cabinet — a microbial contamination caused a Robitussin recall

This concerns lot No. F230169A and expiration 2025-06 of 30 mg Zenzedi tablets. The Zenzedi tablets are yellow, in a hexagonal shape with “MIA” on one side and “30” on the other. The carbinoxamine tablets the pharmacist found in the bottle were white with “GL” on one side and “211” on the other.
If you have a recalled bottle, return it to the pharmacy or medical professional who gave it to you. Inmar Intelligence is handling the recall for Azurity, so ff you have questions about the recall, call Inmar at 877-804-2069, Monday through Friday, 9 a.m. to 5 p.m., Eastern time.
If you experience a medical problem because of this drug, first notify a medical professional. Then tell the FDA via the MedWatch program, either online or by calling 800-332-1088. After that, then you can notify Azurity at 800-461-7449, Monday through Friday, 9 a.m. to 5 p.m., Eastern time.
Wholesale and retail drug sellers can return bottles from the recalled lot to Inmar, 3845 Grand Lakes Way, Grand Prairie, TX 75050.

