Coronavirus test used at the White House might be wrong half the time, researchers claim
A coronavirus test touted by President Trump and used at the White House may miss nearly half of positive cases, according to a study by New York University.
The study looked at Abbott Laboratories’ ID Now molecular COVID-19 rapid test, which Trump hailed as a “whole new ballgame” at a March 30 news briefing because it boasted of delivering positive results in as little as five minutes. But the NYU researchers concluded that when testing swabs were stored before use in a stabilizing solution, the Abbott test missed at least one-third of positive results that were found using a different, more time-consuming procedure, the Cepheid Xpert Xpress SARS-CoV-2 test. Last month, responding to concerns about false negative results, Abbott changed its protocol to recommend using dry nasal swabs, rather than storing them in a solution that could dilute the samples.
But the researchers concluded that actually increased the rate of false negatives to 48 percent.
The research, which has not been confirmed or peer-reviewed, was posted on the website BioRxiv, where COVID-19 researchers have been sharing early results in the hope of speeding innovations for treating the disease. The study compared 101 test samples from patients at New York University Langone Health-Tisch Hospital.
Reached by Yahoo News for comment on the study’s findings, Abbott Laboratories said it was “unclear if the samples were tested correctly.”
“Once again, a study has been conducted using ID Now in a manner that it’s not intended to be used. It’s unclear if the samples were tested correctly and we’re further evaluating these results,” company spokesman Scott Stoffel said in an email. “The outcomes in this paper are inconsistent with any experience that we’ve had with this instrument.”
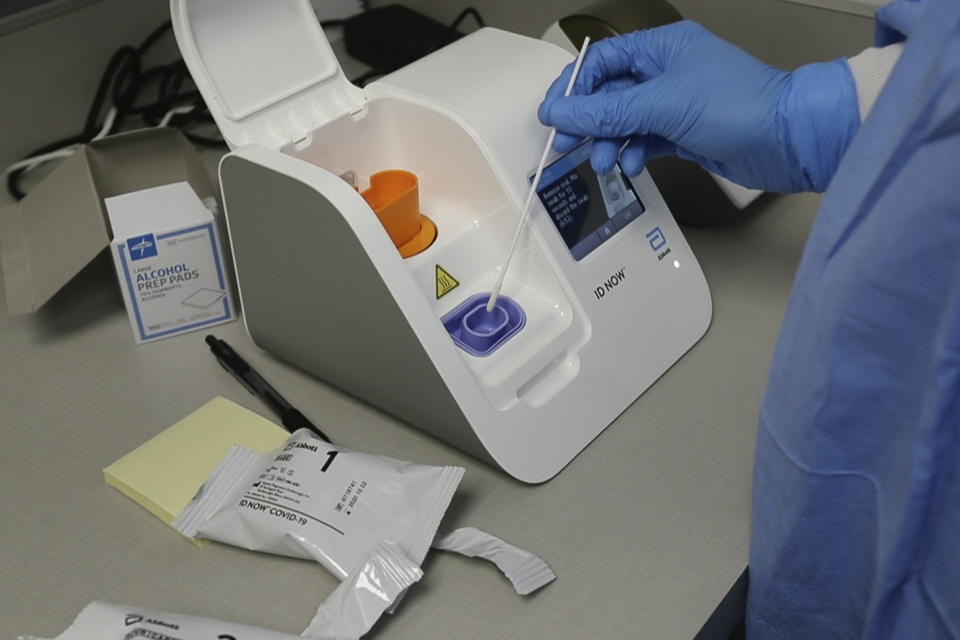
The authors of the paper say they took Abbott’s recommendations into account.
After being granted emergency-use authorization by the Food and Drug Administration on March 27, Abbott has shipped hundreds of thousands of test kits to hospitals, doctor’s offices and pharmacy chains like Walgreens and CVS. The company says it has distributed 1.8 million ID Now tests, and that “the reported rate of false negatives is at 0.02%.”
Accurate testing is crucial as the nation begins easing restrictions meant to slow the spread of the coronavirus, and nowhere has that been more apparent than in the White House itself. Last week, two members of the White House staff tested positive, leading three of the top members of the coronavirus task force to self-quarantine.
At a Friday press briefing at which Trump was asked about Katie Miller, Vice President Mike Pence’s press secretary, who was one of the two staffers to test positive, the president sounded a skeptical note on the value of tests.
“This is why the whole concept of tests aren’t necessarily great,” Trump said. “The tests are perfect but something can happen between the test where it’s good and then something happens and, all of a sudden, she was tested very recently and tested negative. And then today, I guess, for some reason she tested positive.”
At least through last week, the ID Now test was in regular use at the White House.
_____
Click here for the latest coronavirus news and updates. According to experts, people over 60 and those who are immunocompromised continue to be the most at risk. If you have questions, please refer to the CDC’s and WHO’s resource guides.
Read more:




