Cough syrup warning after 66 children die in The Gambia
The deaths of 66 children in The Gambia from acute kidney injuries could be linked to four cough and cold syrups made in India, the World Health Organisation (WHO) has said.
The health body issued an alert on the medications made by Maiden Pharmaceuticals and said they may have been distributed outside the West African country, with global exposure “possible”.
WHO chief Tedros Adhanom Ghebreyesus told reporters the four cold and cough syrups in question “have been potentially linked with acute kidney injuries and 66 deaths among children”.
“The loss of these young lives is beyond heartbreaking for their families,” he added.
The WHO said it is “conducting further investigation” with Maiden Pharmaceuticals and Indian regulatory authorities, and has advised all countries “detect and remove” the products from circulation.
Maiden Pharmaceuticals has been approached for comment.
The Gambia’s Medicines Control Agency sent a letter on Tuesday to health professionals ordering them to stop selling any of the products named.
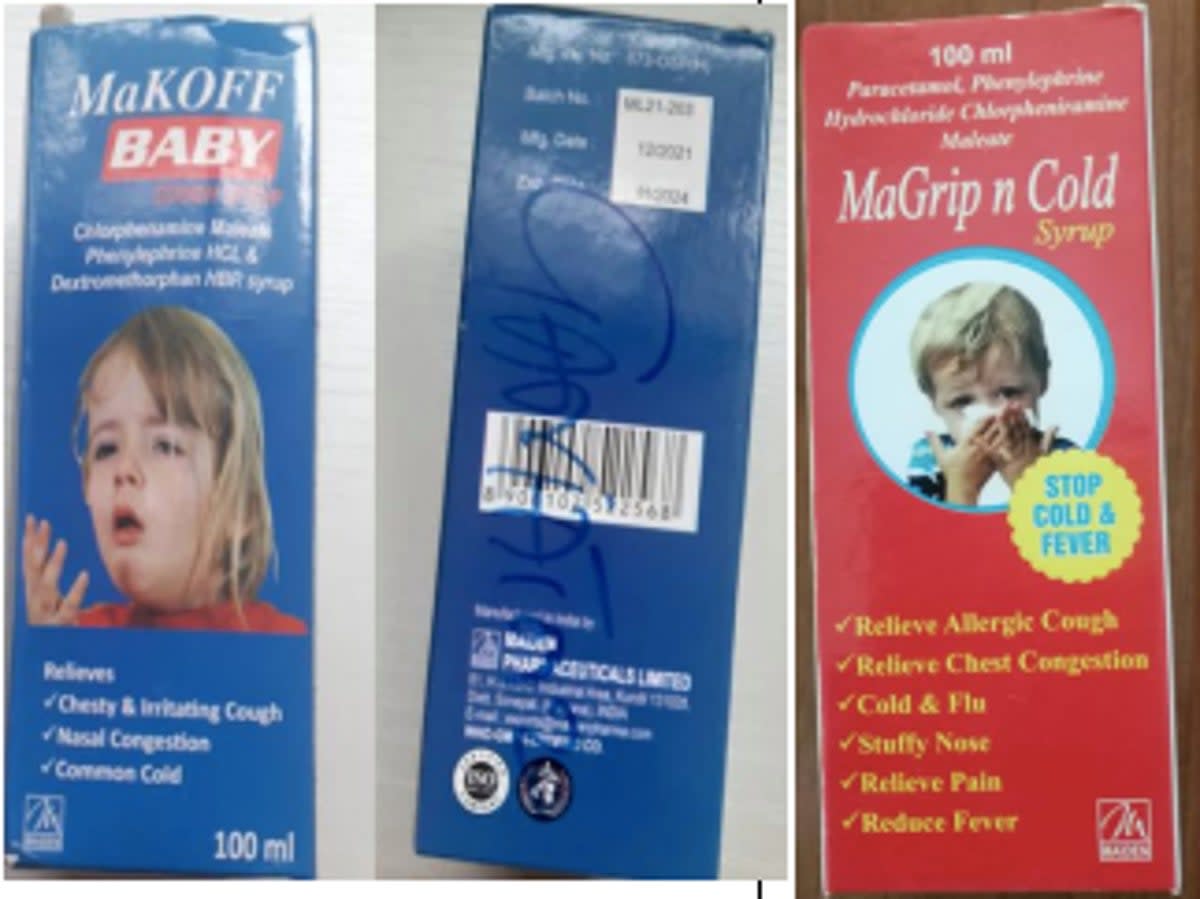
According to the medical product alert issued by the WHO on Wednesday, the four products are Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup.
Samples of each of the cough syrups were tested and identified “unacceptable amounts of diethylene glycol and ethylene glycol as contaminants”, which are toxic to humans and can be fatal when ingested.

The toxic effect “can include abdominal pain, vomiting, diarrhoea, inability to pass urine, headache, altered mental state and acute kidney injury which may lead to death.”
The WHO said that information received from India’s Central Drugs Standard Control Organisation indicated that the manufacturer had only supplied the contaminated medications to The Gambia.
“However, the supply of these products through informal or unregulated markets to other countries in Africa, cannot be ruled out,” the UN agency said in an email.
“In addition, the manufacturer may have used the same contaminated material in other products and distributed them locally or exported,” it warned. “Global exposure is therefore possible.”

