Inexperienced, dubious companies on FDA list to sell coronavirus antibody tests
Investors accused him in court of deceiving them by driving a Rolls-Royce and wearing a gold Rolex to hide his bankruptcy. The Food and Drug Administration barred him from selling dietary supplements after his company failed a string of inspections.
Yet Paul Edalat’s company, Vivera Pharmaceuticals, is one of more than 150 with the FDA’s blessing to sell coronavirus antibody tests – tests that could become vital gatekeepers to reopening America.
For nine critical weeks during the pandemic, the agency exercised little of its power to decide which companies could sell blood tests aimed at detecting whether someone was previously infected. In that vacuum of oversight, USA TODAY — in the most thorough independent review to date — found a nascent industry with inexperienced or dubious companies jockeying to cash in.
For now, public health experts say antibody tests are valuable only for research and identifying plasma donors who could help those who are sick. But if scientists establish that having the virus leads to immunity, the tests could help people decide whether to return to work, socialize or travel. Relying on inaccurate tests poses grave risks.
The FDA’s list of tests has included those from companies with little to no background in medical testing, including one that sells vape pens and one headed by a self-proclaimed technology evangelist. Like Vivera Pharmaceuticals, some have ties to the world of dietary and health supplements; one advertises a male enhancement powder.
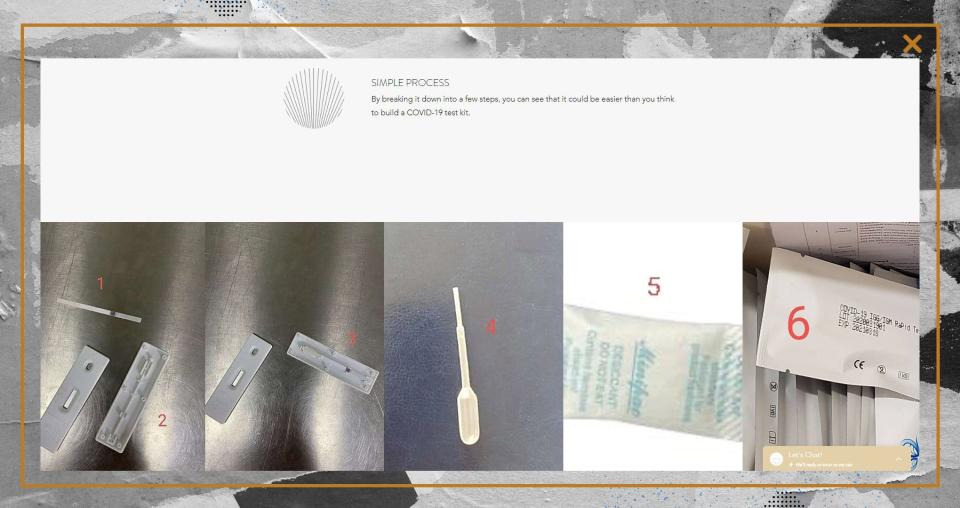
At least five companies have claimed that their tests can be used to diagnose COVID-19, a violation of FDA guidelines. Another offers a do-it-yourself option.
“It could be easier than you think to build a COVID-19 test kit,” it says.
Facing withering criticism, the FDA recently tightened its restrictions, requiring companies to submit data on their test’s accuracy and how it will be marketed. In recent days, about 30 tests have been dropped from the FDA list, some of them voluntarily.

The FDA’s new rules spell out a process for evaluating the tests, but not the manufacturers. As a result, even companies led by CEOs with a history of legal entanglements – including at least one with a criminal past – can sell tests.
Responding to USA TODAY’s findings, the FDA said in a written statement that it takes fraud seriously and “continually monitors and conducts surveillance for fraudulent and inappropriately marketed medical products, including tests.”
“We unfortunately have seen unscrupulous actors marketing fraudulent medical products, including drugs and test kits, using the pandemic as an opportunity to take advantage of Americans’ anxiety,” the agency said.
Scott Becker, CEO of the Association of Public Health Laboratories, said lab representatives were on a conference call with the FDA in March. As the agency outlined its initial plans to allow virtually all comers to sell antibody tests, he said, “You could see the train wreck coming.”
‘FDA confidential’
On social media and in company news releases, Paul Edalat portrays himself as a jet-setting chief executive officer. He has appointed former professional athletes to the advisory board of Vivera Pharmaceuticals.
Currently, the only other products Vivera sells are gel pads to relieve scarring. On March 22, the company applied with the FDA for an emergency-use authorization to sell antibody tests. That approval is far less rigorous than the normal FDA review of new medical products, an approach the agency chose to speed up testing in the pandemic.
Vivera’s chief medical officer, Stephen McColgan, told USA TODAY he expects approval soon.
The FDA now requires all companies to reveal the results of validation tests to the agency. Many companies post accuracy numbers on their websites. Vivera does not – and when asked about the test’s accuracy, McColgan was reluctant to answer.
“It’s all FDA confidential,” he said. “We have a great test, that’s all I can say. There’s no reason your readers need to hear this because they don’t have the level of knowledge to understand.”
Later McColgan offered rough estimates of the test’s accuracy, describing it as “very high.”
Vivera’s antibody test is made by a German company Edalat identified as PharmACT. That company has not applied with the FDA. But because Vivera adds small devices to the test box, including lancets to prick fingers, Edalat contended “the FDA looks at us more as the manufacturer.”
The FDA declined to discuss specific companies but said manufacturers should be the ones applying for emergency-use authorization, naming their distributors in their application.
Edalat has a history with the FDA. In 2014, the agency went to court to stop his company, SciLabs Nutraceuticals, from selling dietary supplements, alleging the products had not been tested to ensure they contained only dietary ingredients. At the time, Edalat said: “We would rather work with the FDA than fight them; they play a critical role in consumer safety.”
Just before the Justice Department issued a permanent injunction on behalf of the FDA, SciLabs went under and Edalat declared Chapter 7 bankruptcy. Months later, four investors allege Edalat persuaded them to put $2 million into a company called Pharma Pak Inc., whose products included the controversial hemp product CBD oil.
The investors filed suit, saying they didn’t know Edalat was not allowed to sell supplements.
“Defendant Paul Edalat is a fraud,” the investor lawsuit alleges. It contends he tried to fool investors with his extravagant lifestyle: staying in luxury suites, “wearing a diamond-studded gold Rolex watch which he brags that he purchased for more than $50,000,” and “driving fancy cars, including two Rolls-Royces, three Lamborghinis, a Land Rover, a BMW, a Ferrari, and a Hummer, among others.”
The suit went before a federal jury, which found that Edalat defrauded and libeled some of the investors. He was ordered to pay them $880,000.
In a case still awaiting trial, Alternate Health Inc. alleges Edalat told a series of lies to ink a 2017 agreement worth $4.2 million to sell a cannabis supplement. The Canadian company claims Edalat said he could mass produce the product and didn’t reveal he was barred from doing so.
SUBSCRIBE: Help support quality journalism like this.
Edalat is pursuing counterclaims against some of the plaintiffs who sued him in the Pharma Pak case, federal court records show. An appeal in that case also is pending. Edalat similarly filed a counterclaim in the Canadian company case, which court records show is awaiting a scheduled Sept. 29 trial date.
When USA TODAY asked Edalat if the FDA had expressed concern about his history, he said, “No, not at all.” The ongoing agency injunction, he said, involved a different branch of the FDA: Supplements are considered food, while antibody tests are medical products.
The FDA declined to say whether such an injunction would prohibit a company from selling an antibody test, stating it would depend on the terms of the enforcement action.
Edalat added that he now suspects there was foul play in the FDA’s inspections of his previous company and he is “investigating the investigators.”
Vivera is distributing samples of its test to places like nursing homes and hospitals, Edalat said. On its website, the company links to a local TV newscast of a firefighter in Hillside, New Jersey, being tested that includes a closeup of a Vivera test box. The doctor who administered that test told USA TODAY Vivera was one of 10 companies that sent him antibody tests to try out.
‘We are businessmen. We see a need.’
Experience in medical testing is not a prerequisite to dive into that world today, thanks to the lax FDA rules for antibody tests.
On its website, Jiangsu Eubo Biotechnology Co. offers male enhancement powders, human growth hormones, anti-hair loss powders, steroids and, until the FDA dropped it from the authorized list on May 21, rapid COVID-19 tests. The company’s website features an illustration of barely dressed male and female fitness models. An email sent to the Chinese company bounced back.

In February, another company, Naturitious, sprang up in California. Owner Danny Xu said he had previously manufactured dietary supplements along with test strips to detect ketosis for low-carb dieters. Producing antibody test kits, he told USA TODAY, is a “pretty similar” process.
Xu said his company has manufactured and shipped 200,000 test kits so far, mostly overseas. The Naturitious website cautions that they are for “professional use only by clinical laboratories and healthcare workers.” It offers an option for customers to buy parts and build their own kits.
Xu said he got into the antibody test business because he wanted to “do something helpful in this pandemic.” But, he said, it’s too complicated for a long-term commitment.
“Working with FDA is hard,” he said. “Dealing with customers is also hard.”
After USA TODAY contacted Xu, a new section appeared on his company’s home page featuring smiling employees in white coats and scrubs alongside placeholder text that reads: “position/role.” The images actually are stock photos, some of them available for sale online.
Pacific Connect Group LLC, based in Hong Kong, is run by a physical therapist for the Chinese Olympic Committee. He teamed up with a recent graduate of Rice University who worked briefly for a hardware design company and started a business to automate airport baggage checking.
Company president Jason Wong and Zach Bielak, his vice president of operations, are now selling N95 and surgical masks.
“We are not doctors. We are not medical specialists. We are businessmen. We see a need,” Bielak said.
The duo pledged not to sell their tests unless they get the FDA’s emergency-use authorization.

The Arizona registration of a new company, Telepoint Medical Services LLC, was approved Dec. 31, the day the World Health Organization was notified about pneumonia cases of unknown cause in Wuhan, China.
Telepoint’s website offers N95 masks, a rapid coronavirus diagnostic test kit, walk-up testing booths and instructions for using its coronavirus antibody test. It gives no information on the test’s accuracy or performance.
Telepoint is run out of a shopping center in Phoenix, according to registration documents filed in Arizona. Larry Witherspoon, listed as the company’s agent, is identified on Telepoint’s website as its owner and managing partner.
Witherspoon’s biography on the website of an organization called the African American Business Foundation says he’s a serial entrepreneur and “technology evangelist” whose ventures have included FaithPhone Wireless and the digital NuGospel Network. He did not respond to messages left with a Telepoint sales employee.
Not a test to diagnose COVID-19
The FDA requires manufacturers to make it clear that antibody tests should not be used to diagnose active COVID-19, the disease caused by the coronavirus. But at least five companies with antibody tests still on the FDA list say their tests can be used that way. Two corrected their claims after being contacted by USA TODAY last week.
Antibodies don’t show up in the blood immediately when a person is infected. So a test that typically uses a nasal swab to gather mucous is used to diagnose COVID-19.
The FDA says when it becomes aware of fraudulent claims regarding antibody tests, it will “take appropriate action, including criminal or civil action.” The agency requires disclaimers by companies, including, “Results from antibody testing should not be used to diagnose or exclude acute SARS-CoV-2 infection.”

Genrui Biotech Inc. of China says on its website that “the disease can be screened and diagnosed” with its antibody test. Another Chinese company, H-Guard Co., says its test for one antibody “is used as a marker for acute infectious diagnosis.”
Boston BioPharma also describes its test as being for diagnostic use. After USA TODAY pointed out the language, a spokesman said the company would revise its wording. Vivera Pharmaceuticals makes the claim, too, although it does include the FDA disclaimer on its site.
Singapore-based Sensing Self presents its test as a pre-screening tool – a finger-prick blood test individuals can administer themselves before deciding if a lab test is warranted. The site blares in a pop-up message that the company has the “world’s first COVID-19 Pre-screening test,” with results in 10 minutes.
On its product page, Sensing Self also said its test is for diagnostic use, and detecting antibodies “is an effective method for the rapid diagnosis of COVID-19 infection.” It adds: “No lab visits, no doctors Just one finger prick of blood.”

Company co-founder and CEO Shripal Gandhi said Sensing Self has sold a “pretty significant quantity across the world” but declined to say how many or where. He said the company has focused on Europe and Asia and now is working with prominent U.S. universities.
Following inquiries from USA TODAY, the company changed the language on its product page.
"We thank you for alerting us,” Gandhi said, “and we have updated the word ‘diagnostics’ to ‘screening.’”
SUBSCRIBE: Help support quality journalism like this.
Company backgrounds not obvious to customers
CoronaCide LLC registered as a company on March 23 in Utah and weeks later in Florida, offering 10-minute antibody tests. Its website says demand is so high that the company accepts only bulk orders.
What potential customers wouldn’t find there is company creator Edward Joseph Eyring II’s past.
Once a colorectal surgeon, Eyring agreed to be barred from renewing his medical license in 2012, about five years after Utah’s professional licensing division investigated him for patient treatment, state records show. Anton Hopen, an attorney representing CoronaCide in a lawsuit against a competitor, said Eyring allowed his medical license to expire.
In one case, rectal and colon surgery for a 37-year-old man was interrupted for emergency repair of a vein lacerated during the operation, the records show. The man underwent two more surgeries by Eyring and died after the third procedure.
Eryring admitted to the state that during those two years he “engaged in unprofessional conduct … when he violated generally accepted professional and ethical standards … by making clinical procedural errors and by providing inadequate medical documentation.”
The attorney general separately investigated Eyring for a series of investment schemes. One involved a $37,000 loan he received from a former patient to help him renew his medical license. Instead, Eyring used the funds for personal expenses, state investigators allege.
In August 2018, Eyring entered a guilty plea to a pattern of unlawful activity, a felony. His prison term was suspended, and he agreed to pay a fine and interest totaling nearly $20,000.
Although CoronaCide is included on the FDA’s list of antibody test manufacturers, Hopen said in an email it’s actually a distributor. He said CoronaCide had registered as a distributor "for complete transparency."
In some cases, the websites of companies on the FDA list offer clues to customers only because the information they provide is so sparse.
The website for Carlsbad, California-based Axium Bioresearch says it is a leading provider for toxicology, women’s health and health prevention testing. Last month, its website said nothing about a coronavirus antibody test.
After a USA TODAY reporter asked about the omission, the company updated its website to feature a microscopic image of the coronavirus and a link to inquire how much the test costs. Friday, around the time of a USA TODAY interview with company president Sergius Albert Salvatore, access to the website changed again and required a password.
Salvatore said he learned about medical tests by working with research labs in China. His small company receives antibody test components from partners there, he said.
Axium adds other components to create rapid-result testing kits for sale through distributors to hospitals and medical facilities in Latin America, Salvatore said, but the customers won’t buy unless the tests have FDA approval.
“In no way do I say I’m a scientist,” he said. “I have scientists who are on board in China.”

In Michigan, the state attorney general says VitaStik, which was taking orders for at-home antibody tests, was running a scam. The FDA had never allowed at-home tests and specifically forbade them on March 20.
On April 1, VitaStik – which specializes in vaping devices for vitamins and essential oils – was ordered to stop.
“False reliance on whatever test strips you are selling could have deadly consequences for both those who buy them and their loved ones,” Michigan Assistant Attorney General Darrin Fowler wrote.
Ten days after the attorney general’s order, VitaStik’s owner set up a new company, Vita Testing, to sell rapid antibody tests. The FDA added it to the list of companies that could sell in the U.S.
Vita Testing’s website offered 100 tests for $3,500. It warned they could be sold only to labs certified to perform complex testing and associated medical providers, but it offered to notify people when at-home tests are available.
“We are listed on the FDA.gov website, and so is our test,” the company assured.

Vita Testing was among those dropped from the FDA list on May 21. The next day its website went offline.
In an email to USA TODAY, company owner Alfred Santos didn’t explain why he created the new company after Michigan ordered his previous one to stop selling tests there. But he said none of the tests sold in Michigan was shipped and the money was refunded.
A spokesman for the Michigan Attorney General’s Office, Ryan Jarvi, said: “The purchase our special agent had made under a different name was refunded,” and the office planned no further action, aside from sharing information with “other law enforcement agencies that have expressed interest in this target.”
Santos wrote in his email that he is “ACTIVELY working” with the FDA and still hopes to get emergency-use authorization for his antibody tests to be used in labs, health care settings and, one day, homes.
David Heath is a reporter on USA TODAY’s investigations team. Contact him at DHeath@usatoday.com. Donovan Slack and Kevin McCoy are reporters on USA TODAY’S national team. Contact them at DSlack@usatoday.com and KMcCoy@usatoday.com.
This article originally appeared on USA TODAY: Coronavirus antibody tests include some dubious, inexperienced firms

