COVID-19: Is my at-home test still good? What you need to know

COVID-19 has crept back into southwest Ohio and northern Kentucky as two omicron subvariants have become the predominant strain of infection locally. But if you're looking to shake the dust off of your at-home rapid antigen tests to use before a late-summer vacation or a visit to grandma, there are some factors to consider.
The first is effectiveness. Though studies regarding the accuracy of at-home tests were performed specifically on previous variants, experts say the sensitivity on the tests should be enough to pick up on BA.4 and BA.5, two highly infectious omicron subvariants currently spreading throughout Ohio and the Cincinnati region.
A study of the effectiveness of BinaxNow, a commonly used at-home rapid antigen test, found that the BinaxNow test accurately registered a positive result in 95% of samples that were also found positive by a polymerase chain reaction (PCR) test.
"What we don't know is, is this as good with the BA.4, BA.5 omicron as with BA.1 and BA.2?" said Dr. Stephen Feagins, chief clinical officer of Mercy Health Cincinnati. "This is just the best that we know. We think even with BA.4, BA.5, that 95% is probably somewhere similar."
BA.5 variant: Now almost half, if not more, of new COVID-19 cases in Ohio
COVID-19 in Cincinnati: CDC flashes a caution sign about rising levels in area counties
Nose or throat swabs?: Advice from doctors so you don't waste that COVID-19 testing kit
The tests, if used within proper expiration dates, will provide accurate results regardless of variant, said Amanda Carter, a spokeswoman for Hamilton County Public Health. The department recommends continuing to utilize the tests for a number of situations you might find yourself in as cases and hospitalizations rise.
"Any time you can take an additional step to mitigate the spread, whether it's taking a proactive test before you see someone who's high risk, whether you're coming from a county that has high community spread, we recommend tests for anyone who wants to be cautious and take prevention steps," Carter said.

FDA extends expiration dates
Expiration dates are an important second factor. Though you may have a test at home that appears to have expired, you'll want to check the extended expiration dates issued in May. The date printed on the box could be the wrong expiration date, given the extensions.
The shelf life of the following at-home tests have been extended by the FDA.
BinaxNow AG Card Home Test (extended from 12 to 15 months).
BinaxNow Antigen Self Test (extended from 12 to 15 months).
CareStart Antigen Home Test (extended from 9 to 12 months).
Flowflex Antigen Home Test (extended from 12 to 16 months).
Celltrion DIaTrust AG Home Test (extended from 12 to 18 months).
Detect COVID-19 Test (extended from 6 to 8 months)
iHealth Antigen Rapid Test (extended from 9 to 12 months).
SCoV-2 Ag Detect Rapid Self Test (extended from 10 to 13 months).
Pilot At-Home Test (extended from 6 to 9 months).
Expiration dates for these tests remain the same, according to the FDA.
BD Veritor At-Home Test (6 months).
Cue Test for Home and Over the Counter (4 months).
Ellume Home Test (12 months).
Genabio Rapid Self Test Kit (18 months).
Lucira Check-It Test Kit (6 months).
MaximBio ClearDetect Antigen Home Test (6 months).
Inteliswab Rapid Test (9 months).
OHC Antigen Self Test (8 months).
Indicaid Rapid Antigen At-Home Test (12 months).
QuickView At-Home Test (12 months).
Clinitest Rapid Antigen Self Test (11 months).
Speedy Swab Rapid Antigen Self Test (6 months).
Rapid Antigen Test Card (6 months).
The FDA recommends ditching tests that have expired beyond the extension dates. The risks are that the tests can become dried out and over time and won't give an accurate test result.
"Anytime beyond the extended period, is time to get a new test," Carter said.
Feagins agreed, noting that it makes sense to have peace of mind given the availability of at-home testing.
"After doing the research and checking the actual expiration date and realizing that it has passed even the extended expiration date, there's a ton of these tests out there, go ahead and get a new one," he said.
Underreported testing
Last Thursday, the Cincinnati region's community risk level was upgraded to "high," triggering an indoor mask recommendation by the Centers for Disease Control and Prevention. An accurate picture of how much community spread exists however has become much more challenging since widespread use of at-home tests began.
As more individuals utilize at-home testing, officials worry many of the results go unreported and an accurate tabulation of community spread is unattainable.
At a COVID-19 press briefing in May, Dr. Ashish Jha, the federal COVID-19 response coordinator described the challenge the tests have presented.
"Home tests are great, by the way," Jha said. "I’ve been a huge fan of home tests for the last two years. But what that means is we’re clearly undercounting infections — undercounting cases."
Instead of using the number of cases as a reliable figure, many communities now turn to trends in hospitalizations.
Though intensive care unit beds filled with COVID patients remains low locally, the Cincinnati region has seen an increase in hospitalizations since the beginning of the month. As of last Thursday, there were 197 COVID patients hospitalized in the region, a number that rose by nearly 80 patients in two weeks, according to the Health Collaborative's Situational Dashboard. The region's medical surgical beds are 97% full, while ICU beds are 93% occupied, according to the latest data.
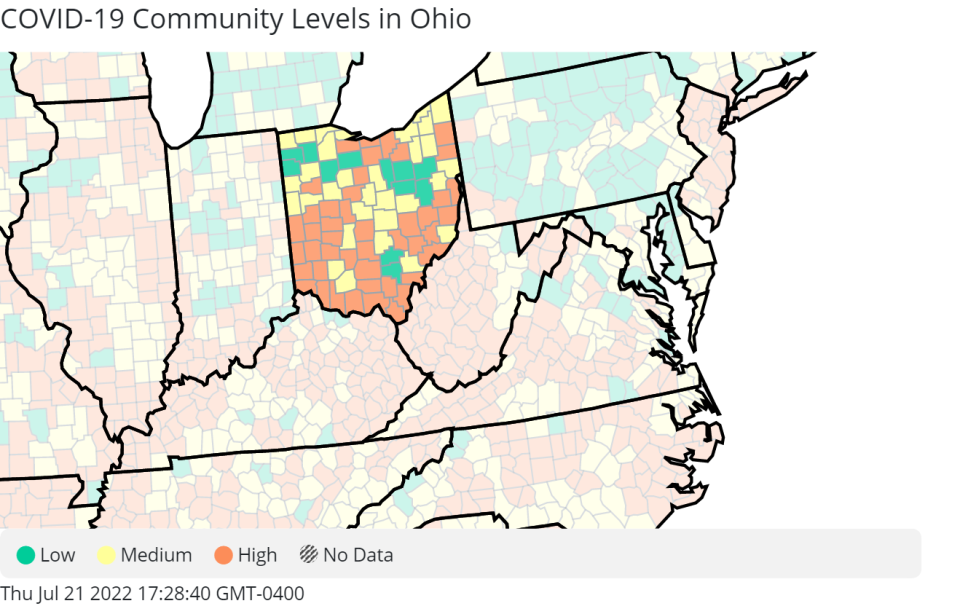
The upgrade to high community level, which is determined by looking at hospital beds being used, hospital admissions, and the total number of new COVID-19 cases in an area, recommends a number of preventative steps.
Wear a well-fitting mask indoors in public, regardless of vaccination status (including in K-12 schools and other indoor community settings).
If you are immunocompromised or high risk for severe disease: Wear a mask or respirator that provides you with greater protection; Consider avoiding non-essential indoor activities in public where you could be exposed; Talk to your healthcare provider about whether you need to take other precautions (e.g., testing); Have a plan for rapid testing if needed (e.g., having home tests or access to testing); Talk to your healthcare provider about whether you are a candidate for treatments like oral antivirals, PrEP, and monoclonal antibodies.
If you have household or social contact with someone at high risk for severe disease: consider self-testing to detect infection before contact; consider wearing a mask when indoors with them.
Stay up to date with COVID-19 vaccines and boosters.
Maintain improved ventilation throughout indoor spaces when possible.
Follow CDC recommendations for isolation and quarantine, including getting tested if you are exposed to COVID-19 or have symptoms of COVID-19.
In-person tests can be arranged by visiting the Health Collaborative's Test and Protect Cincy website, where you can find a schedule and information about appointments.
To help agencies get a better understanding of community spread, local health departments have asked individuals who do utilize at-home tests, to self report positive tests. Some tests given through a doctor, clinic, or health department are proctored, meaning a staffer watches the test be administered and the result through a smart phone or app.
But at-home tests purchased at a store or obtained by the government aren't proctored. If you take an at-home test and receive a positive result, here is how you can report it to your local jurisdiction.
Hamilton County Public Health isn't tracking results of at-home COVID-19 tests. If you are located in the city limits of Cincinnati, the Cincinnati Health Department has three options for self reporting: visiting the department's designated online questionnaire through a browser, scanning the QR code to get to the questionnaire, or by calling in results to 513-357-7462.
Individuals not located in Cincinnati can report their result to their doctor, Carter said.
This article originally appeared on Cincinnati Enquirer: How long does a COVID-19 at-home test last?

