So When Will a COVID-19 Vaccine Be Available?
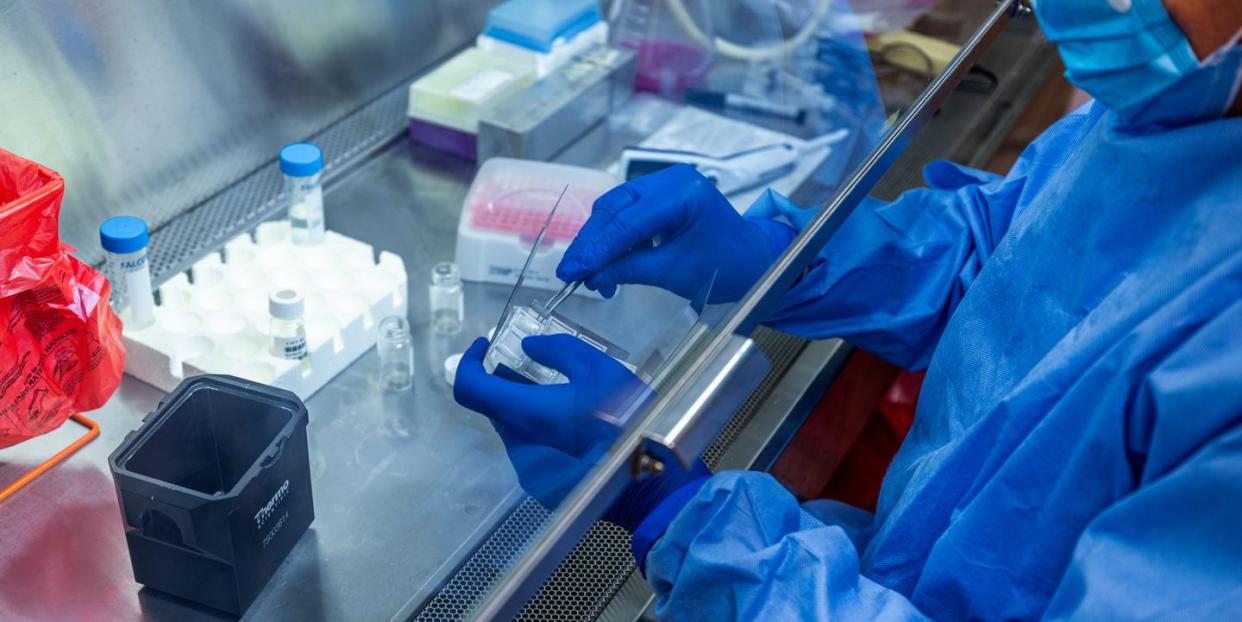
Louis Falo still has the scar. It’s on the bicep of his left arm, an oblong mark left when a needle scratched into his flesh a tiny bit of toxin—just enough to kickstart an immune response, but not enough to make him sick.
This is how doctors across the United States once vaccinated generations of people against smallpox. The process was so effective that by the early 1950s, scientists declared the deadly viral disease eradicated in North America. Two decades later, America’s doctors stopped administering smallpox vaccines to the general population altogether. Dr. Falo’s scar is the inexpensive price paid to ensure he never got the virus. It would also prove to be an inspiration when he turned his attention toward hopefully eradicating a new deadly virus.
Since authorities in China reported a peculiar form of pneumonia to the World Health Organization in late 2019, novel coronavirus and the disease it causes, COVID-19, have ricocheted around the world. In the U.S., the country currently with the most cases, the virus has already infected more than one million people in two months, killing more than 58,000—about 46,000 more than those who died in the U.S. during the swine flu pandemic of 2009-2010 (as of April 29).

Vanquishing COVID-19 has so far relied on a disruptive protocol of social distancing, quarantines, testing, mask-wearing, and handwashing. Effective drugs and treatments, the sort that can get us all out of our homes again, are still being investigated for their potency. But a vaccine, the metaphorical uppercut that could knock out COVID-19 before it wreaks any more havoc, would bring this all to a swift conclusion.
Just one problem: Making a vaccine is anything but swift. The fastest-ever approved vaccine, for mumps, took four years.
Working tirelessly despite the pandemic-level pressure and the daunting timelines are people like Louis Falo, M.D., Ph.D., an immunologist and skin dermatologist at the University of Pittsburgh. It was there that Dr. Falo and a team of 10 other scientists and physicians began working in January 2020 to discover a vaccine for COVID-19—one they could apply to the skin, like the smallpox vaccine, using only a small patch resembling a bandage.

“It’s a unique combination of excitement and stress,” Dr. Falo says. “You have the excitement of the hope that you actually might be able to do something to stop the pandemic. And at the same time, you have the stress of not knowing whether or not you actually [can].”
What usually takes years to unfold is being rapidly truncated into weeks. In early April, Dr. Falo and his colleagues unveiled PittCoVacc, a vaccine they say generates COVID-19-fighting antibodies. That was only the beginning. What happens over the next 18 months could spell the end of the pandemic.
A Viral Locksmith
Almost as quickly as scientists realized they had a new disease on their hands, they had the genomic sequence of SARS-CoV-2, the coronavirus that causes COVID-19. Shortly after the new year, on January 11, Chinese scientists published online the machine-readable schematic of 29,903 nucleic bases that make up the new virus’s genetic RNA. Dr. Falo and his University of Pittsburgh colleague, Andrea Gambotto, M.D., were waiting for this moment.
“He actually was reading the newspaper, saw that the sequence was now publicly available from an article he read, and then he went online and got it,” Dr. Falo recalls. “We were off and running from that point.”
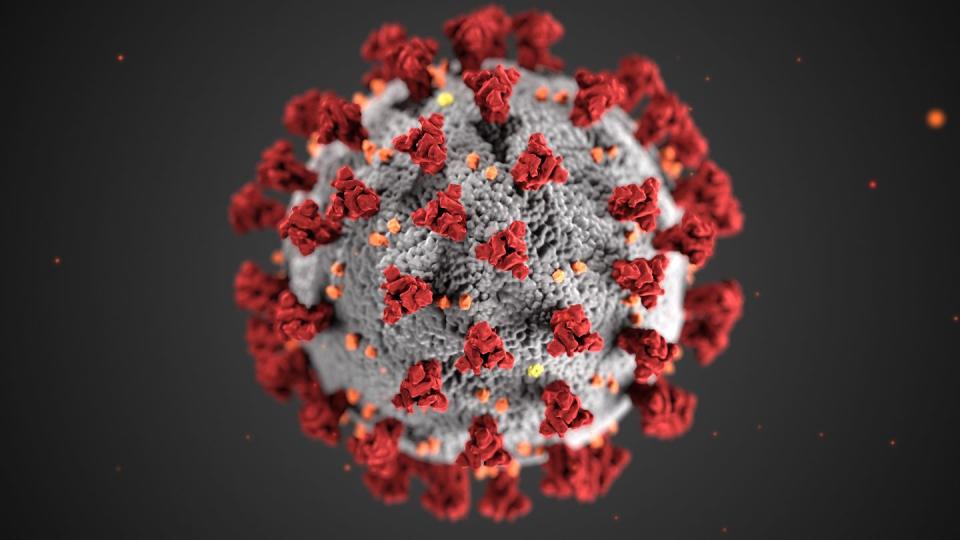
Having the genomic sequence meant they could begin analyzing specific proteins that might serve as useful antigens—portions of the virus that might trigger the immune system, training the cells in our bodies to recognize COVID-19 virus as a hostile invader and develop antibodies to not only terminate the enemy, but also grant long-lasting immunity to it.
By now the image of the magnified rendering of the new coronavirus is ubiquitous: a menacing sphere covered in protruding red spikes. Call it a viral locksmith. The spike latches onto one of many receptors affixed to the outer membrane of our cells, effectively unlocking them. At that point, the virus deposits its RNA, hijacks our cellular machinery, and cranks out copies of itself.
WATCH: THE MAKING OF A COVID VACCINE AT UNIVERSITY OF PITTSBURGH
The team at Pitt had previously studied the SARS virus in 2002 and the MERS virus in 2014, two other spike-laden coronaviruses. They figured the S-protein that composes the spikes on the outer envelope of the COVID-19 virus was the most promising antigen target. Within days of the genomic sequence being published, Dr. Gambotto’s team working on computers optimized the published S-protein genetic sequence to efficiently produce pieces of the protein in cultured cells.
Targeting a virus with such precision is a 21st-century invention, the result of computers becoming powerful enough to quickly and cheaply sequence the genetic codes of pathogens. It allows vaccine development to move at lightning speed, which helps explain the 76 different COVID-19 vaccines currently in progress. And although Dr. Falo’s team has worked quickly, their vaccine is not the first to enter a clinical trial.
Starting on March 16, a potential vaccine was injected into 45 healthy adults in Seattle and Atlanta. The concoction was brewed by Moderna, a biotechnology firm based in Cambridge, Massachusetts, and developed in tandem with scientists from the National Institute of Allergy and Infectious Diseases. (Its director, Anthony Fauci, M.D., is America’s pandemic guru.) Moderna’s vaccine is an example of mRNA technology: It encodes a mutated spike protein by shooting a snippet of genetic RNA into the body. In other words, the vaccine provides instructions to the body’s cells so they can produce an antigen internally.
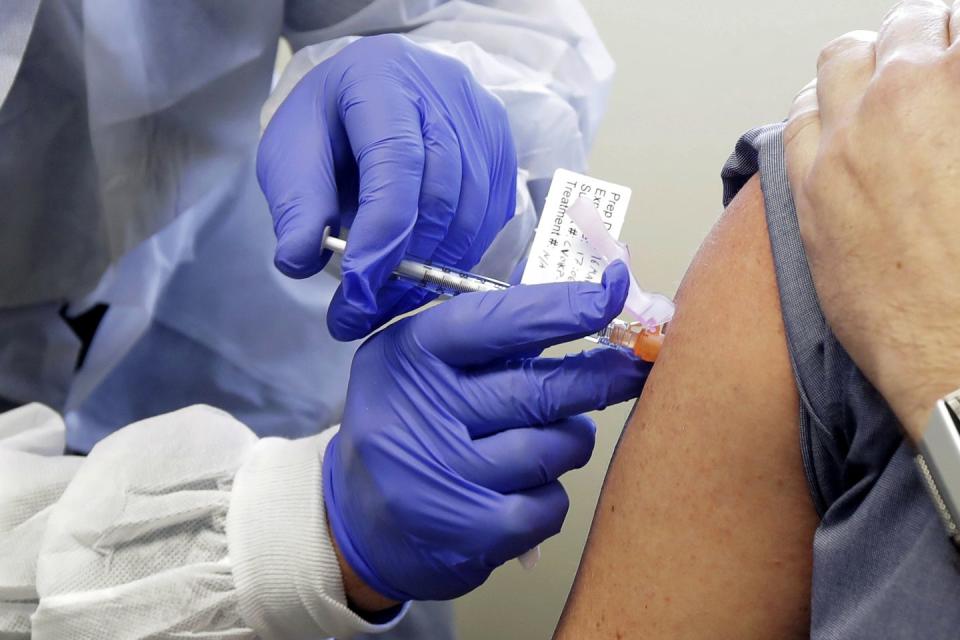
“We’re just using one protein that is important for inducing an immune response: the spike protein. We essentially are optimizing the immune response to that target protein,” says Kizzmekia Corbett, Ph.D., lead scientist for coronavirus vaccine research at NIAID. “Our hypothesis is that with this vaccine strategy, we’ll get better antibodies.”
Outside the laboratory setting, however, RNA vaccines are unproven: Because they’re so new, they’ve never been licensed for use on humans.
A pandemic certainly loosens the parameters of experimentation. Even so, Dr. Falo’s group, from the beginning, was set on using a type of vaccine technology that’s been around since the 1980s—one that produces antigens externally, in a lab. It was during the 1980s that vaccine developers began producing subunits of virus, just pieces of a larger virus (like a sliver of its membrane protein) synthetically assembled from proteins and sugars. These subunits are the antigen. Once injected, they induce the immune system to manufacture antibodies. Some flu vaccines are the quintessential examples of this approach.

With the gene sequence of the novel coronavirus’s spike protein in hand, the Pitt team decided an effective vaccine could be made from a portion of viral S1 protein (a subunit of the spike protein). By injecting this into a person, the thinking goes, the immune system will produce antibodies to recognize and bind to the spikes of the coronavirus, blocking and shutting down the locking mechanism before the virus has a chance to attach to and hijack our cells.
Complex Choreography
Dr. Falo’s team set a timeline of two weeks, with the aim of performing trials of their vaccine in mice in early February. A complex choreography ensued: Even immunologists and virologists have to keep distance during a pandemic. The University of Pittsburgh vaccine team of 11 divided into smaller subgroups.
“This was a shift-type effort. In the event that somebody came down with symptoms, we wouldn’t all be quarantined,” says Dr. Falo. Optimizing time became a Herculean task. He remembers working one 18-hour stretch, only pausing to exit the lab and slug some coffee, grab a snack, or catch a short nap.

One group was responsible for producing large quantities of the viral antigen (the S-protein), which is done in cell factories. Picture a miniature skyscraper of 10 plastic trays stacked on top of each other, each filled with millions of human cells (specifically human embryonic kidney cells commonly used in biotechnology to produce proteins and viruses). In a process called transfection, lab scientists used an electronic pulse to open pores into those cells that let in the viral gene that makes the S1 protein. After a couple of days, the scientists were able to harvest a soupy fluid rich in coronavirus S-protein pieces.
Group number two, led by Dr. Falo, was responsible for devising the delivery system. After all, what good is a viral antigen if you can’t get it into the body? This is where Dr. Falo’s background in dermatology—and his knowledge of smallpox vaccination—played a big role.
Typically vaccines are delivered via needle directly into the muscle, but the skin also generates a potent immune response—and contains more immune cells than muscle tissue. The human dermis is awash with dendritic cells that act like scouts, moving around the skin and searching for foreign pathogens. When they discover one, they break off a piece of it, then move quickly into the body’s lymphatic system, traveling in earnest to present the invader to B and T cells, which generate the antibody response. Dr. Falo’s thought was simple: Forget injecting the antigen into the blood stream. Stick it into the skin instead, and let the dendritic cells do all the heavy lifting.

“The skin is your first line of defense, constantly getting bombarded with bacteria and viruses,” he says. “Our goal was to take what we learned from smallpox and develop technology that was able to reproduce that, but in a much more controlled, efficient fashion.”
Having a doctor etch viral antigen into a person’s skin is cumbersome, not to mention a lousy way to combat a pandemic. While he wasn’t expecting a new coronavirus to shut down the world, Dr. Falo was nonetheless searching for an easier way to deliver skin inoculations. For the last nine years, he and fellow Pitt researchers have been developing what he calls a microneedle array: a small patch that resembles a Band-Aid covered in 400 microscopic needles, and something that a person could theoretically create at their local makerspace. The molds for these arrays can be designed in computer software, then manufactured by a high-resolution 3D laser printer down to the nanometer.
Meanwhile, the needles themselves are made from sugar substances capable of dissolving into the skin. With S-proteins produced and microneedle-array molds printed, Dr. Falo and his fellow scientists put the final touches on their vaccine. The bits of viral protein were mixed in with a sugary solution, which was poured into the molds. Two sessions in the centrifuge first pulled the antigen down into the needles before hardening them. Afterward, team members used tweezers to pull the microneedle arrays from the molds, almost like breaking ice out of its tray. Left over was a square patch, filled with COVID-19 vaccine, that could be comfortably applied to a person’s arm, and removed in under 15 minutes.

“The needle is actually the vaccine, and the delivery method is the dissolving of the needle,” says Dr. Falo. “After that, you just take off the patch and throw it away.”
At the end of those two weeks, the vaccine team applied patches to mice in the lab on the backs of their ears. While Dr. Falo and his team had their results early, they weren’t published in The Lancet until the first week of April. In mice, PittCoVacc produced a surge of COVID-19 antibodies, enough to neutralize the virus, within two weeks.
Race Against the Clock
There are two additional benefits to the patch delivery system. The first is that it’s easy to produce. The second is that the patches don’t need to be kept cold. Once the antigen is incorporated and solidified in the microneedle array, it’s stable at room temperature.
“It’s a relatively straightforward technology that doesn’t use specialized equipment, so one person in our lab today can make hundreds of these,” he says. “And these can be stored and shipped just like Band-Aids.”
Whether the vaccine that the Pitt develops ultimately makes it to the world is now a matter of time. And time is of the essence. It seems likely that a vaccine won’t become available until 2021 at the earliest—not soon enough to stave off a second surge of infections some scientists predict will occur later this year.

“We have a virus that’s basically embedded into society at this point, and it’s not going away until we stop transmission completely,” NIAID’s Dr. Corbett says. “The only way to do that is via vaccine.”
The progress the U.S. makes over the coming months will be vital. While many countries have embarked on the same race as American scientists, it’s likely that whatever vaccine works against the novel coronavirus won’t be produced in quantities large enough for global distribution right away.
“Even with extraordinary international collaboration among multiple countries, it could be years before a vaccine is produced at a scale sufficient to help the entire world,” wrote former FDA commissioner Scott Gottlieb in The Wall Street Journal the last weekend of April.
At the same time, any vaccine that’s administered must be safe. This is why all vaccines go through three phases of trials, the first being a test for safety with a relatively small sample size, like the trial currently underway for Moderna’s vaccine in Seattle and Atlanta with 45 people. As trials progress, the sample is gradually increased, until you enter a phase-three trial with thousands of participants. The goal, at that point, is to judge overall efficacy.
Still, other drawbacks might get in the way of even the best-engineered vaccine. Many aspects of this new coronavirus remain unknown, and its ability to mutate could be its most pernicious. Influenza, for instance, mutates regularly, which is the reason flu shots are administered every year. Whether that’s the case with the novel coronavirus remains to be seen.
“We can only guess from what we have from the past,” Dr. Falo says. “The past coronaviruses, MERS and SARS, have very, very low mutation rates. That doesn’t mean this one will be, but from what we’ve seen, I think we’ve got a stable virus here.”

Yet a pre-print research study by one of China’s top scientists concluded that the virus’s ability to mutate had been greatly underestimated, and that such mutations could explain the varying rates of infection—and severity—among different populations across the world.
That risk is one that Dr. Falo’s team, as well as other vaccine researchers, are carefully considering as they race against the clock. According to Dr. Fauci, Moderna’s vaccine is on track to be distributed publicly anywhere from a year to 18 months from now.
“It’ll take a few months to get the data to where we’ll feel confident to go to the phase two, and then a few months from now we’ll be in phase two and I think we’re right on target for the year to year and a half,” he said earlier this month during a White House pandemic press conference.
That timeline is what Dr. Falo and the University of Pittsburgh’s vaccine team are working with right now. And while Dr. Falo knows the importance of clinical testing, he’s eager to get PittCoVacc into people.
“It’s the team that made this happen,” he says. “We can envision shipping boxes and boxes of these all over the world.”
Within the next couple months, they plan to begin a human clinical trial to test the safety of their vaccine, as soon as they get approval from the Food and Drug Administration. So for now Dr. Falo is still working in the lab, spinning centrifuges, harvesting viral proteins, taking tweezers to microneedle arrays—and racing to release what may wind up being one of the most important vaccines on the planet.
You Might Also Like

