COVID home tests recalled after bacteria that could lead to ‘serious illness’ was found
Pilot COVID-19 At-Home Test Kits, at least 516,000 of which went to CVS Health and Amazon, have been recalled across the United States by SD Biosensor after “potentially harmful bacteria were found” in a tube with liquid included in the test.
“Direct exposure to the liquid in the tube through misuse or spillage could potentially lead to serious illness,” the company-written, FDA-posted recall notice says.
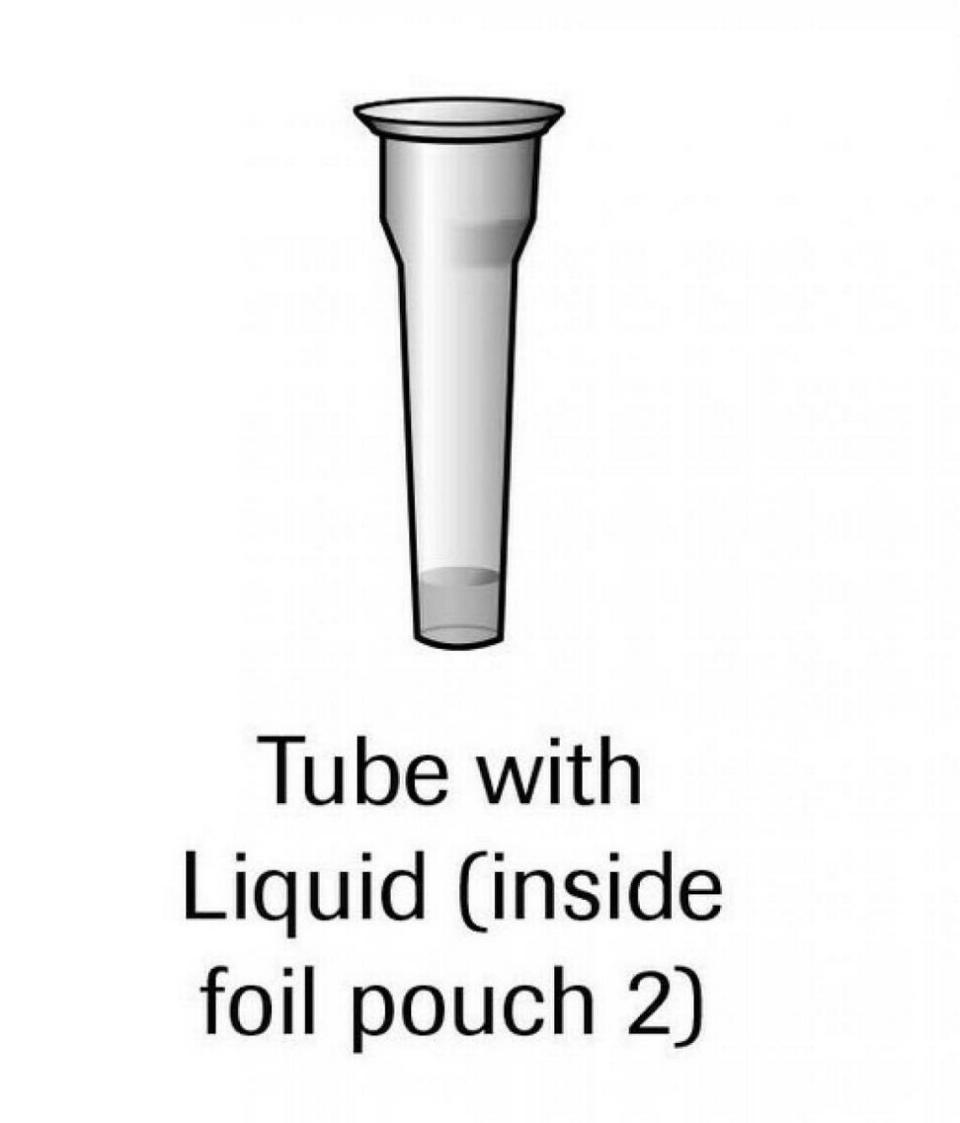
If that liquid gets on your skin or in your eyes, use copious amounts of water to flush the area. If you feel a continuing irritation, head for a medical professional.
The FDA said Roche Diagnostics distributed the test to other distributors and retailers, two of which were CVS Health (500,000) and Amazon (16,000). Users are asked to throw out the tests if they’re in one of the following 44 recalled lot numbers:
53K38N1T1; 53K38N2T1; 53K38N3T1; 53K38N4T1; 53K38N5T1; 53K38P1T1; 53K38P2T1; 53K38P3T1; 53K41T5T1; 53K41X1T1; 53K41X2T1; 53K41X3T1; 53K4211T1; 53K4212T1; 53K4213T1; 53K4221T1; 53K4222T1; 53K4223T1; 53K4224T1; 53K4225T1; 53K4231T1; 53K4232T1; 53K4233T1; 53K4261T1; 53K4262T1; 53K4271T1; 53K4272T1; 53K4273T1; 53K4274T1; 53K4291T1; 53K4292T1; 53K42A1T1; 53K42A2T1; 53K42A3T1; 53K42E1T1; 53K42G1T1; 53K42G2T1; 53K42H1T1; 53K42H2T1; 53K42L1T1; 53K42L2T1; 53K4361AC; 53K4362AC; and 53K4392AC.
READ MORE: Here’s why a national discount store chain recalled 7 different kinds of Advil
The lot numbers can be found on the side of the packaging.

If you have any questions about this recall, including asking about getting replacement tests, contact Roche online or by calling 866-987-6243. Press option No. 1.
If you’ve had any medical problems from this or any other medical test, first see a medical professional. Then let the FDA know via its MedWatch Adverse Event page or by filling out a form you can get by calling 800-332-1088.

