A COVID vaccine for children? Kansas City area volunteers needed for Pfizer trials
Children as young as 6 months of age could get COVID-19 vaccines as part of a nationwide pediatric trial led locally by Children’s Mercy, the hospital announced Wednesday.
The study will evaluate the safety and tolerability of the Pfizer-BioNTech vaccine, and whether it can stimulate an immune response. The vaccine could be available to babies and children up to age 11 in the general public by early 2022, according to Pfizer.
More than 5,000 area children have already enrolled in the trial at Children’s Mercy, hospital officials say. But because some might eventually choose not to participate because of the required time commitment and what’s involved in the study, researchers would like more families to consider participating to find those perfect fits.
“This is a much-needed study that will help us continue gathering evidence on the efficacy of the already developed vaccine on our younger populations, including children and infants,” lead researcher Dr. Barbara Pahud, director of research, infectious diseases, at the hospital, said in a statement.
“While the virus has affected more adults, children have also died and been hospitalized, and our goal is that COVID-19 will become another vaccine-preventable disease which will save lives.”

Pfizer-BioNTech and Moderna are both studying vaccines for children as young as 12, and one of those could be available as early as this summer, according to the American Academy of Pediatrics.
And, like Pfizer, Moderna is also conducting trials for a vaccine for children 6 months through 11, and health officials estimate it will be available in late 2021 or early 2022, the pediatrics group says.
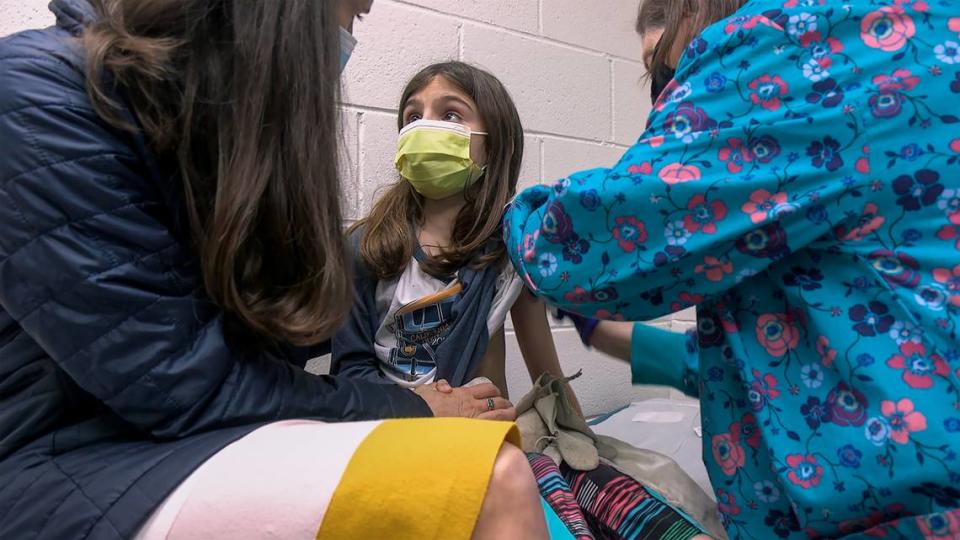
The first phase of the Pfizer study at Children’s Mercy focuses on finding the correct dose. In the next two phases, some participants will receive the vaccine and some a placebo.
The young participants will have their blood drawn before receiving two doses, about a month apart, and again a week after receiving the last dose.
Participants will also have a nose swab before each of the doses to test for COVID-19 antigens. Six months after receiving the second dose, participants who were given the placebo can choose to receive the vaccine.
Children 7 and older will have to consent on their own to participate, and any participant can drop out at any point. Pahud emphasized that participating in the trial is “100% voluntary.”
“This trial requires a number of shots, blood draws and nasal swabs, and our goal is to ensure children feel comfortable and safe while samples are collected,” she said.
Families can sign up online. The link is on the Children’s Mercy website, news.childrensmercy.org. Information about compensation for participants was unavailable Wednesday.
Children’s Mercy is also hosting several COVID-19 vaccine clinics this month on both sides of the state line for residents 16 to 22. Health experts encourage teenagers attending proms this year to get vaccinated before the party. For information on how to sign up go to childrensmercy.org or call 816-234-3700.

