CytoDyn Reports Top-Line Data From Phase II Coronavirus Study
CytoDyn Inc. CYDY announced top-line results from its recently completed, randomized, double-blind, phase II study evaluating the efficacy and safety of leronlimab in patients with mild-to-moderate symptoms caused by COVID-19 infection.
Shares of the company fell 13.5% following the news as the investors were not impressed with the results. However, shares of the company have soared 316% year to date against the industry’s decline of 5.6%.

In the study, the primary endpoint showed early clinical improvement in symptom score at day 3 in patients receiving leronlimab. At day 3, more patients treated with leronlimab reported improvement in total clinical symptom score compared to the placebo group (90% on leronlimab arm versus 71% on placebo).
Leronlimab also demonstrated statistically significant improvement versus placebo in key secondary efficacy endpoint, National Early Warning Score 2 (NEWS2) scale. NEWS2 (the latest version) is being used as an endpoint in several other COVID-19 studies. It measures clinical parameters, including respiratory rate, oxygen saturation, supplemental oxygen, temperature, systolic blood pressure, heart rate and level of consciousness.
Treatment with leronlimab demonstrated reductions in both serious adverse events as well as predictors of pulmonary collapse in patients with mild-to-moderate COVID-19.
The company will request immediate approval of leronlimab for this population of COVID-19 patients, not only in the United States but also in the U.K. and other countries around the world.
Leronlimab is an investigational, humanized IgG4 mAb that blocks CCR5, a cellular receptor that is important in HIV infection, tumor metastases and other diseases, including NASH. The FDA has granted a Fast Track designation to leronlimab for two potential indications of deadly diseases —one as a combination therapy with HAART for HIV-infected patients and another for metastatic triple-negative breast cancer.
In July 2020, the company reported impressive results from the phase II COVID-19 study. The results showed that 39% of patients in placebo arm as compared to only 14% in leronlimab arm reported serious adverse events (SAEs), which were unrelated to leronlimab.
At this time, there are minimal treatment options for COVID-19. A potential approval of leronlimab will be a huge breakthrough to meet this unmet medical need.
Meanwhile, several marketed drugs like Incyte and Novartis’ NVS JAK1/JAK2 inhibitor, Jakafi; AstraZeneca’s AZN BTK inhibitor, Calquence; and Amgen’s AMGN PDE4 inhibitor, Otezla, among others, are being evaluated to treat respiratory complications associated with COVID-19, which is the need of the hour.
CytoDyn currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
CytoDyn Inc. Price
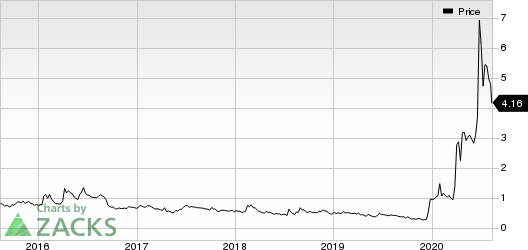
CytoDyn Inc. price | CytoDyn Inc. Quote
Zacks Top 10 Stocks for 2020
In addition to the stocks discussed above, would you like to know about our 10 finest buy-and-hold tickers for the entirety of 2020? Last year's 2019 Zacks Top 10 Stocks portfolio returned gains as high as +102.7%. Now a brand-new portfolio has been handpicked from over 4,000 companies covered by the Zacks Rank. Don’t miss your chance to get in on these long-term buys.
Access Zacks Top 10 Stocks for 2020 today >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Novartis AG (NVS) : Free Stock Analysis Report
Amgen Inc. (AMGN) : Free Stock Analysis Report
CytoDyn Inc. (CYDY) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
