New data says the latest COVID variant is less worrisome than first imagined. Vaccines are helping protect against it.
The newest COVID-19 variant isn't as scary as it seemed at first and fall booster shots should protect against it and all the other currently circulating variants, new data suggests.
In a clinical trial, the updated vaccine generated a nearly 9-fold increase in neutralizing antibodies against the BA.2.86 variant, according to data released early Tuesday from vaccine-maker Moderna. Although extremely rare in the United States, the new variant has a number of mutations in the spike protein targeted by vaccines, which made experts worry that shots and previous infections wouldn't be protective.
But three studies released since the weekend, along with Moderna's new data, suggest the variant, nicknamed Pirola, isn't so bad — at least for now, said Dr. Eric Topol, professor and executive vice president of Scripps Research in La Jolla, California.
"It's been downgraded from a hurricane to not even a tropical storm. We're lucky," Topol said. "This one could have been really bad."
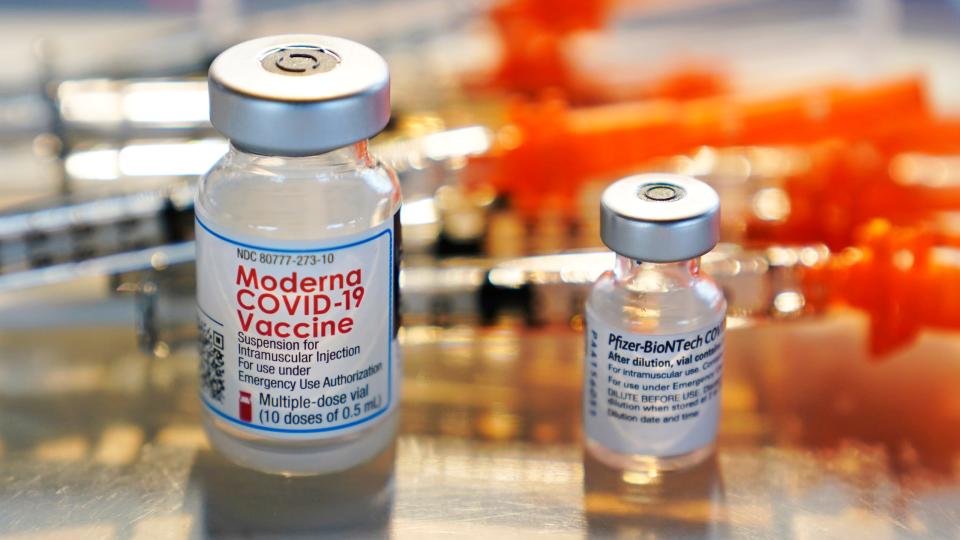
Current state of the pandemic
COVID-19 infections have been rising since early July, data from the Centers for Disease Control and Prevention shows. Hospitalizations are up nearly 16% and deaths nearly 17% in the week that ended Thursday, compared to the week before, though totals remain well below previous peaks.
"It's not looking so good from the standpoint of infections," Topol said.
According to CDC data, EG.5, sometimes called Eris, accounts for more than 21% of COVID-19 infections in the United States; FL.1.5.1 for more than 14%; and two XB.1.16 variants a total of 18%. A wide variety of variants make up the balance.
The current wave is probably being driven by fading protection against the virus and the lack of prevention measures, like masking and social distancing, Topol said. Officials are urging people to get boosters this fall, particularly if they are over 65 or have health issues.
Current vaccines do not provide long-lasting protection against infection with COVID-19, but young, otherwise healthy people have generally been protected against severe disease from either vaccination or previous infection. Vaccines have been shown to reduce the risk of long COVID-19, in which people have lingering symptoms such as brain fog and crushing fatigue for months or even years after an infection.
Everyone can benefit from a vaccine, Topol said, but the size of the benefit is much greater for those who are older or have health issues.
"Immunity to this virus after 6 months is not very good for high-risk people," he said.

Where the vaccines stand
In June, a Food and Drug Administration advisory panel recommended that this fall's booster target the XBB.1.5 variant.
Panel members said they didn't expect the variant, which dominated infections this spring, would still be in full force this fall. But since they couldn't predict the future, they hoped XBB.1.5 would be close enough to whichever variant took over that the vaccine would be protective.
So far, that gamble looks like it's paying off.
Pfizer's laboratory research shows its updated vaccine also effectively prevents severe disease from variants XBB.1.16., XBB.2.3 and EG.5.1.
Both Pfizer-BioNTech's and Moderna's vaccines, which are based on messenger RNA technology, are expected to become available in the U.S. almost immediately after a September 12 CDC advisory committee meeting, which will recommend how the boosters should be used.
Novavax's vaccine, which uses tiny particles along with an adjuvant to amplify its effects, is likely to arrive later in the fall, because of its longer production time.
Last week, the European Medicines Agency recommended authorizing Pfizer-BioNTech's booster in Europe. The European Commission is reviewing the recommendation and is expected to make a final decision in the coming weeks, with the companies ready to ship the vaccine immediately after authorization.
What we know about BA.2.86
Pirola, officially called BA.2.86, has only been spotted a few times so far in the United States, but it's already made the World Health Organization's list of "variants of concern."
One of more than 1,500 known variants of Omicron, BA.2.86, has between 34 and 36 different mutations in the spike protein compared to XBB.1.5. As of late last week, Texas was the fourth state to report a case of BA.2.86, according to Houston Methodist Hospital, and it had been identified in other countries, including Israel, Denmark, and South Africa.
But three new studies out earlier this week suggest BA.2.86 is not as infectious as previous variants or likely to undermine vaccines and previous immunity. The first, posted online and not yet peer-reviewed, showed that the variant does not get into cells very well, suggesting it will not cause a tremendous number of infections.
A second study, also in pre-print, shows that the variant does not evade the immune system as much as had been feared. People in Sweden who had been exposed to XBB.1.5 seemed to have good protection against BA.2.86.
A third, again, not yet peer-reviewed, showed that despite all its mutations, BA.2.86 does not seem to be good at avoiding immune protections. The Moderna data, though lacking the context of how the 9-fold increase in antibodies compares to other variants, also indicates the vaccine will remain effective.
The combination of all these new data points, suggests BA.2.86 won't be a major threat in its current state, Topol said. But he and others worry it could add more mutations over time, becoming more contagious and more likely to avoid both immune protections and vaccines.
"In the months ahead, it could be that BA.2.86 picks up some more mutations and behaves differently," he said. "But right now, things are looking pretty good from the standpoint of the booster."
Contact Karen Weintraub at kweintraub@usatoday.com.
Health and patient safety coverage at USA TODAY is made possible in part by a grant from the Masimo Foundation for Ethics, Innovation and Competition in Healthcare. The Masimo Foundation does not provide editorial input.
This article originally appeared on USA TODAY: COVID-19 variant BA.2.86 not as worrisome as first imagined: Data

