Doctors urge NHS to restart animal organ transplants on humans
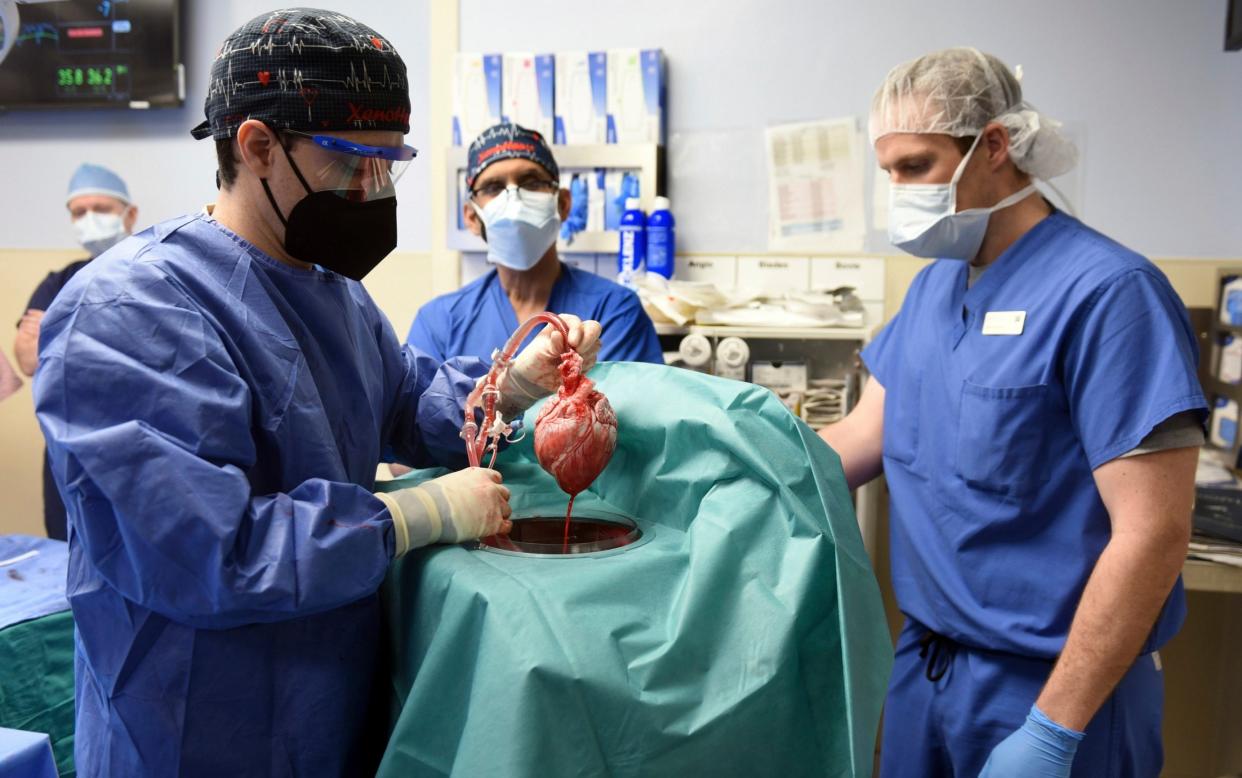
Doctors and experts have called for the NHS to renew its plans for patients to receive animal organ transplants after shelving nascent legislation 16 years ago.
The recent success of two pig transplants into human patients in the US has led to calls to overhaul the myriad regulatory obstacles facing patients and scientists in the UK.
Experts have criticised the “shortsighted” decision to disband the UKXIRA, the UK Xenotransplant Interim Regulatory Authority, in 2006 when the prospect of xenotransplants, using animal organs or tissues in people, were a “long way in the future”.
The body was set up in 1997 to help British policymakers navigate the stormy waters of the emerging field and dissolved due to a lack of development in the field.
But now, the use of genetically modified organs to treat humans is no longer a pipe dream and clinical trials may soon be a reality. However, the UK risks being left behind unless it can update its antiquated rules and regulations.
Prof Sara Fovargue, a University of Sheffield scholar who wrote a book on xenotransplantation in 2011, told The Telegraph: “Xenotransplantation raises particular legal and ethical issues which require careful consideration by a specialist regulatory body, such as the now disbanded UKXIRA.
“It is presently unclear whether if a xenotransplant was performed in the UK, it would be regulated as an experimental treatment or procedure, or as clinical research.
“It is vital that the regulatory pathways are clear, so that potential patients or research participants are appropriately protected.”
Prof Sheila McLean, a University of Glasgow ethics in medicine expert and a former member of the UKXIRA, added: “When UKXIRA existed, the concept of xenotransplantation of whole organs seemed a very long way in the future. But now, although still experimental, it has become closer on the horizon and inevitably throws up moral and ethical issues which require informed and expert analysis.”
Prof Marie Fox, the chairman of Law at the University of Liverpool, said: “It was a very short-sighted decision to disband UKXIRA, as I argued at the time. The UK’s history of regulating the issue has been very reactive, when it was absolutely clear that at some point these procedures would be back on the agenda.”
The US is the current global superpower when it comes to making xenotransplants a reality, due to looser ethical and legal regulations than in the UK, as well as bountiful private investment.
As a result, a patient in Maryland with terminal heart failure recently received a pig heart in a world-first experiment. The operation was a last resort and required emergency approval from the US Food and Drug Administration, but this was granted and has since been a success.
In another world first, a scientific paper published this week revealed that a pair of genetically modified porcine kidneys were successfully transplanted into a 57-year-old dirt bike enthusiast from Alabama who was left brain dead following a tragic accident.
A game of legistlative gymnastics
In contrast, there are no experiments ongoing in the UK due to the need for academics to placate a host of different regulatory bodies and committees, with no sole body having overarching authority.
While theoretically trials could go ahead within the NHS, it would involve a complex game of legislative gymnastics, and one nobody has thus far managed.
Prof Gabriel Oniscu, the president-elect of the European Society of Organ Transplantation, said: “What we need to do is to make sure [xenotransplantation] is adequately regulated and ethically and morally acceptable for the public. I think those discussions need to restart now.”
A spokesman for the NHS’s Health Research Authority told The Telegraph: “Under current rules, a study involving xenotransplant of GM [genetically modified] organs in humans could apply for permission to start in the NHS. An independent Research Ethics Committee would consider the risks against the potential benefits, and whether the study would be legal, ethical and fair.
“Our expert volunteers are the people to consider these risks, but without seeing a study proposal it’s impossible to comment further on decision making.”
A spokesman for the Medicines and Healthcare Products Regulatory Agency (MHRA), said: “Whilst transplant procedures with pig hearts are not human medicinal products and are therefore not regulated by the MHRA, no such transplants would take place in the UK without extensive ethical and safeguarding consideration.”

