Eyedrop safety: How to avoid unsafe products this allergy season
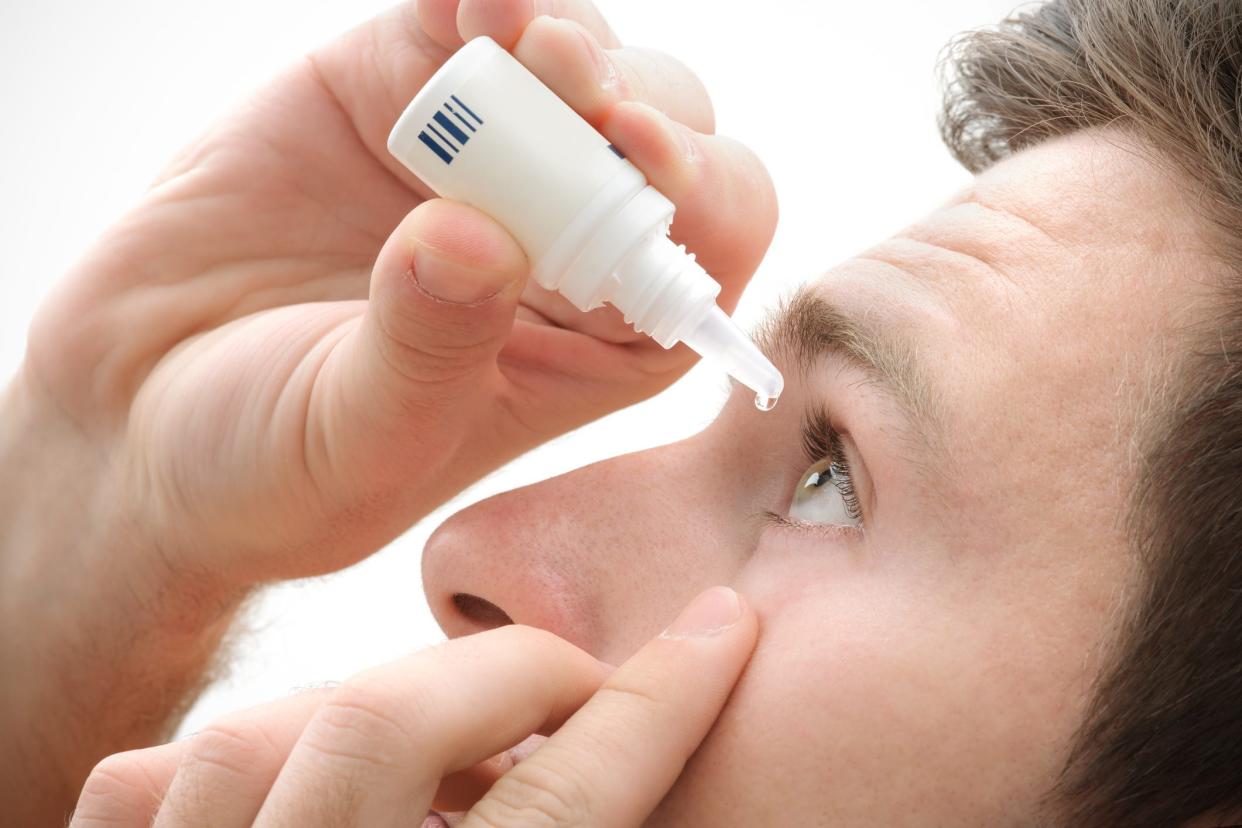
Corrections & Clarifications: Natural Eyes Pink Eye Relief and Dryness Relief, eyedrops made by The Relief Products brand, are preserved, sterile products sold in sterile eye drop bottles and do not require multi-dose preservative-free droppers or single use vials.
Oregon’s grass season is usually at its peak from Memorial Day through the Fourth of July, with high grass pollen in the Willamette Valley. If you have seasonal allergies, you already may be experiencing itchy, watery or red eyes.
But many eyedrops purchased online and some in stores may be unsafe for your eyes due to incorrect packaging or improper manufacturing processes, health care professionals warn.
Before purchasing over-the-counter eyedrops, check to see if the product is registered with the Food and Drug Administration (FDA). Even if a product is registered with the FDA, it may be unsafe to use.
Homeopathic or preservative-free products sold in stores and online can be unsafe if they are not packaged in the correct container.
There are multiple safety boxes a product must check off, said Dr. Sandra Brown, an ophthalmologist and medical advisor for the Dry Eye Foundation, a nonprofit organization to improve the quality of life of those with dry eye disease.
An example is EzriCare Artificial Tears, a product registered with the FDA as an over-the-counter product. The FDA recalled the eye drops, and two others Feb. 1 and issued a warning after the Centers for Disease Control and Protection conducted an investigation into drug-resistant infections in people.

The Dry Eye Foundation has a list of eyedrops to avoid, found at eyedropsafety.org, categorized by severely dangerous products to those that are concerning to the organization.
“It's really time for American consumers to be aware of these products, which seem to them generally to be harmless and beneficial [but] can actually be harmful and deadly,” Brown said.
What to look for when purchasing eye drops
The No. 1 thing to look for in eyedrops is FDA registration, Brown said. The Food and Drug Administration does not approve over-the-counter products, but these products can be registered with the FDA if they meet their guidelines.
The FDA has a database of registrations at accessdata.fda.gov.
“If it's not registered, the FDA has not heard of it,” Brown said. “You definitely should not buy it and use it.”
If a product is registered with the FDA, it also will have a National Drug Code (NDC) on the box. The 10-digit code appears in this format: NDC XXXX-XXXX-XX.
Most non-FDA registered eyedrop products are purchased online, Brown said. Some companies market their products to look similar or identical to credible brands, but those products are not always manufactured or packaged safely.
One example is the product “Refresh Tears Plus,” which looks like the widely available Refresh Brand.
Refresh sells safe, FDA-registered products such as Refresh Tears and Refresh Plus. But “Refresh Tears Plus” is not registered with the FDA, and is on the eyedropsafety.com list of unsafe products because the product marketed in Australia and New Zealand "is being resold illegally in the US, like many other eye drops on this list."
Brown said products sold online may be less expensive than those in stores. But buyers should verify it is an identical product from the same seller.
Regular eyedrop bottles pose a risk for contamination in preservative-free products. They should be in a multi-dose preservative-free dropper or in single-use vials.
Generic brands sold in stores such as Walmart's Equate are safe for use and can be a less expensive option, Brown said.
She also said anyone who wears contacts should be cautious when purchasing eyedrops. The packaging should either say “for contacts” or be a contact rewetting product sold by a known brand.
“The key message is manufacturing eyedrops is a serious business and companies have to take it seriously,” Brown said. “It's not just an eye drop.”
Sydney Wyatt covers healthcare inequities in the Mid-Willamette Valley for the Statesman Journal. Send comments, questions, and tips to her at SWyatt@gannett.com, (503) 399-6613, or on Twitter @sydney_elise44
The Statesman Journal’s coverage of healthcare inequities is funded in part by the M.J. Murdock Charitable Trust, which seeks to strengthen the cultural, social, educational, and spiritual base of the Pacific Northwest through capacity-building investments in the nonprofit sector.
This article originally appeared on Salem Statesman Journal: Avoid unsafe eyedrop brands online and in stores

