Facility with barefoot workers made CVS, Target, Walmart eye drops in second recall
The second run of eye drops recalled “after FDA investigators found insanitary conditions” at the manufacturing plant includes store brands of CVS, Rite Aid, Target and Walgreens.
Here’s what to know
Why are these eye drops being recalled?
Kilitch Healthcare India Limited’s facility in Navi Mumbai is supposed to be a “sterile drug manufacturing facility,” but a series of October FDA inspections found numerous violations of sterility at the plant run by Managing Director Paresh Mehta.
In one of the most glaring instances described in the 20-page report, the inspector wrote “personnel are required to remove company uniform and don headgear, shirt, trouser, booties, hand gloves and footwear prior to entering the Grade C area. Review of the closed circuit TV footage from Oct. 3 and 4, 2023, showed personnel working in the Grace C area where materials are passed into the Grade B filling area.
“Operators did not wear the specified gowning, gloves and were working barefoot as they transferred materials into the Grade B area for filling line. The deputy manager of production confirmed that this is their standard practice.”
The FDA recommended on Oct. 25 that Kilitch recall eye drops from this facility, then issued a warning to the U.S. public about various brands of eye drops on Oct. 27. Three sellers didn’t wait for Kilitch, pulling the trigger on recalls on Nov. 2.
Kilitch made Wednesday’s recalls, 21 days after the FDA’s recommendation. Velocity Pharma distributed the eye drops in the United States for Kilitch.
“For those patients who use these products, there is a potential risk of eye infections or related harm,” the recall notice states. “These products are intended to be sterile. Ophthalmic drug products pose a potential heightened risk of harm to users because drugs applied to the eyes bypass some of the body’s natural defenses.”
What eye drops are in this recall?
All of the below eye drops with expiration from this month to September 2025 are in Wednesday’s recall by Kilitch. The National Drug Code (NDC) number can be found on the box or bottle.
CVS Health: Lubricant Eye Drops in a 15 ml bottle, NDC No. 76168-702-15 and in a twin pack, NDC 76168-702-30; Lubricant Gel Drops in a 15 ml bottle, NDC No. 76168-704-15, and in a twin pack, NDC No. 76168-704-30; Multi Action Relief Drops in a 15 ml bottle, NDC No. 76168-706-15; Mile Moderate Lubricating Eye Drops in a 15 ml bottle, NDC No. 76168-711-15; Lubricant Gel Drops in a 10 ml bottle, NDC No. 76168-712-10; Lubricant Eye Drops in a 10 ml bottle, NDC No. 76168-714-10, and in a twin pack, NDC No. 76168-714-20.
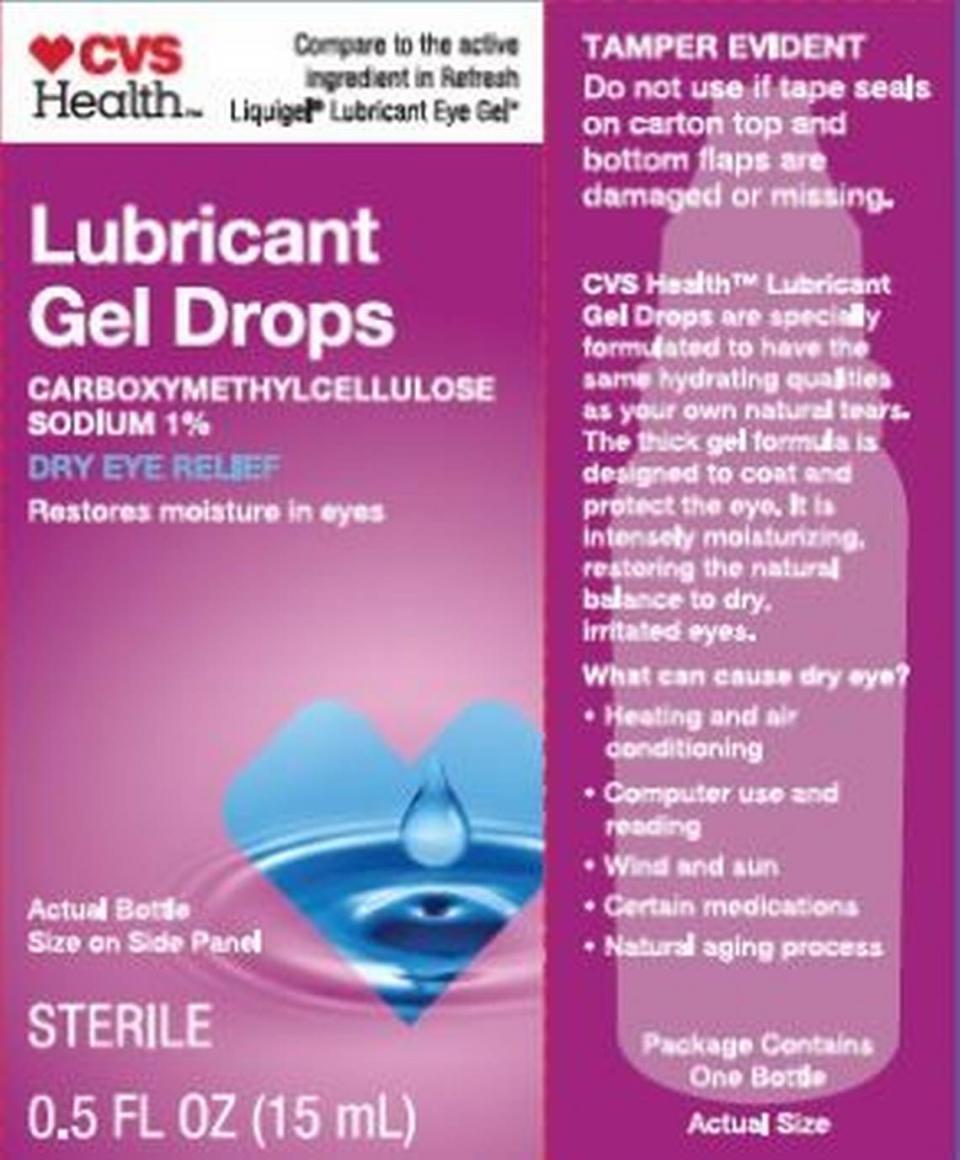
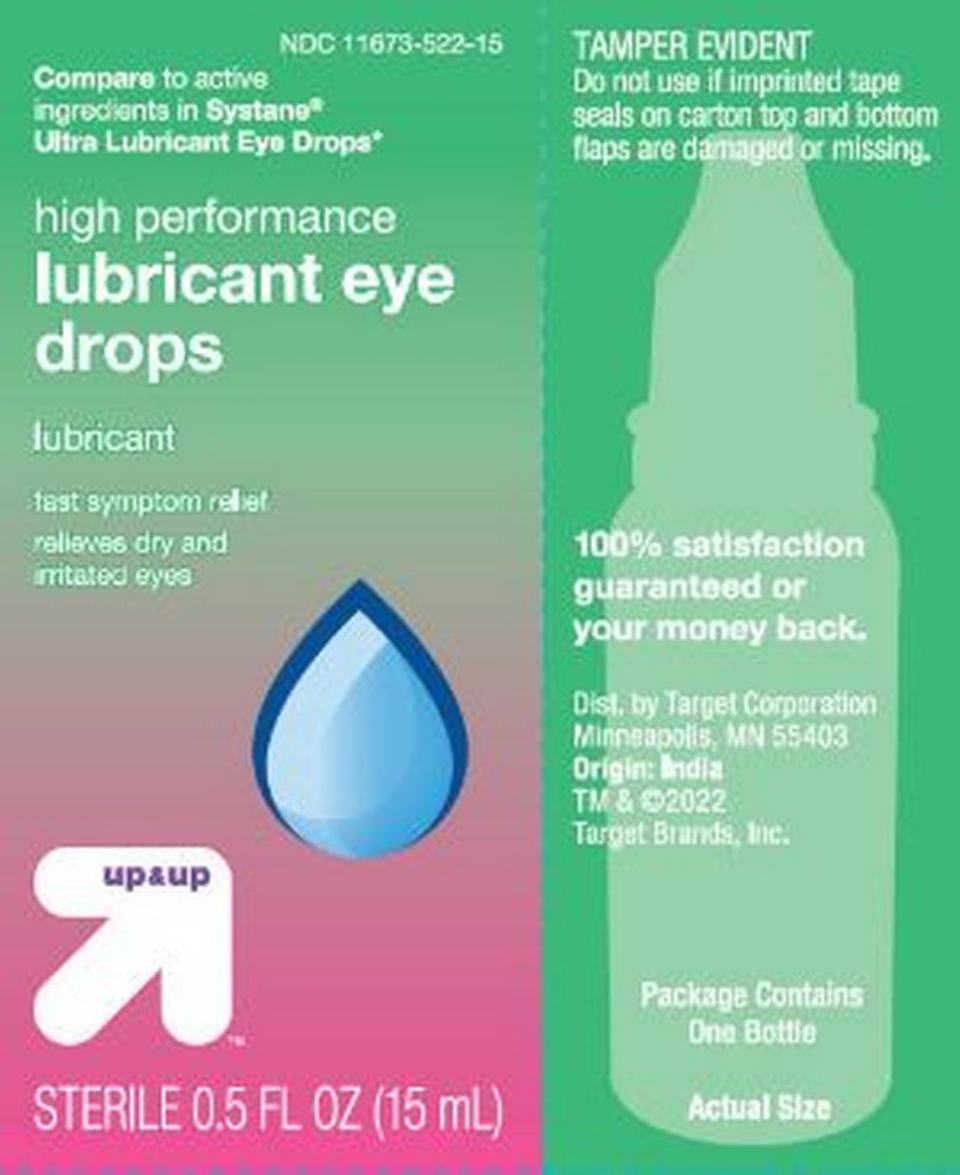
Target’s Up & Up: High Performance Lubricant Eye Drops in a 15 ml bottle, NDC No. 11673-522-15 and in a twin pack, NDC No. 11673-522-30; and Dry Eye Relief, twin pack of 15 ml bottles, NDC No. 76168-800-30.
Walmart’s Equate: Hydration PF Lubricant Eye Drops in a 10 ml bottle, NDC No. 79903-168-01.

Rite Aid: Multi-Action Relief Drops in a 15 ml bottle, NDC No. 11822-2254-3; Lubricating Gel Drops in a 10 ml bottle, NDC No. 11822-4540-3; Lubricating Gel Drops in a 15 ml bottle, NDC No. 11822-9706-5; Lubricating Eye Drops in a 10 ml bottle (twin pack), NDC No. 11822-4811-3; and Lubricating Eye Drops in a 15 ml bottle (twin pack), NDC No. 11822-9707-5.

Leader: Eye Irritation Relief in a 15 ml bottle, NDC No. 70000-0087-1; Dry Eye Relief in a 10 ml bottle, NDC No. 70000-0088-1 and in a 15 ml bottle, NDC No. 70000-0089-1; Lubricant Eye Drops in a 10 ml bottle, NDC No. 70000-0587-1, in a 15 ml bottle, NDC No. 70000-0090-1, and in a twin pack of 15 ml bottles, NDC No. 70000-0090-2.
Rugby: Lubricating Tears Eye Drops in a 15 ml bottle, NDC No. 0536-1282-94; and Polyvinyl Alcohol 1.4% Lubricating Eye Drops in a 15 ml bottle, NDC No. 0536-1325-94.

Velocity Pharma: Lubricant Eye Drop in a 10 ml bottle, triple pack, NDC No. 76168-502-30.

What should you do if you have any of these eye drops?
▪ Refund: Don’t use the eye drops in the recall. Return them to the seller for a refund.
▪ Questions: If you have questions about these recalls, email the distributor at regulatory@velocitypharma.com or the manufacturer at regulatory@kilitchhealthcare.com.
▪ Notification: Any health issues from these products should be taken to a medical professional first. Then, notify the FDA via the MedWatch program, either online or by getting a form to fax or mail, online or by calling 1-800-332-1088

