FDA warns Ozempic users after seizing thousands of units of counterfeit weight loss drugs
The U.S. Food and Drug Administration is advising extra caution after seizing thousands of units of counterfeit Ozempic, according to a notice issued Thursday.
The imitation medications have been associated with five cases of illness so far, said the agency, but none have been reported as serious. Those who used counterfeit doses have reported adverse reactions consistent with the authentic product, including nausea, vomiting, diarrhea, abdominal pain and constipation.
While the FDA and Ozempic's manufacturer, Novo Nordisk, have already collected and are testing thousands of samples, they have warned that some counterfeit products may still be available for purchase.
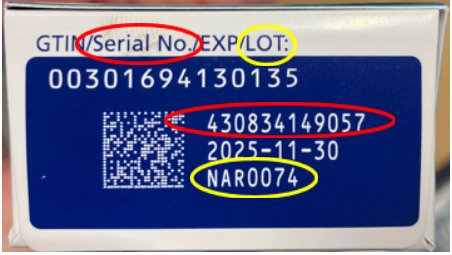
Because of this, wholesalers, retail pharmacies, health care practitioners and patients alike are advised to check any semaglutide they have received to ensure they are not part of the bogus supply, which is labeled with lot number NAR0074 and serial number 430834149057.
Celebs and Ozempic: Oprah Winfrey's revelation about using weight loss drugs is a game changer. Here's why.
Keep an eye out for counterfeits

The FDA and Novo Nordisk are currently testing collected samples to determine their contents, safety and efficacy. Thus far, they have determined that the products' pen labels, health care professional and patient information and the carton in which the medication comes were all counterfeit.
Importantly, the needles were also found to be counterfeit, meaning their sterility cannot be confirmed and may pose an increased risk of infection to patients.
Anyone currently in possession of a semaglutide product is advised to take a close look before using, selling or otherwise distributing it to confirm not only the lot and serial numbers, but also the labeling and packaging it comes in. The FDA encourages consumers to compare photos posted to their website of authentic Ozempic packaging to the products in their supply for differences in color, labels and overall appearance.
The agency also reminded pharmacies to purchase Ozempic only through authorized Novo Nordisk distributors and patients only to obtain it through a prescription from a state-licensed pharmacy. Everyone handling the product is advised to look it over carefully for signs of tampering or counterfeit.

What is Ozempic (semaglutide)?
What is Ozempic: You've heard of Ozempic, but do you understand how it works?
Weight loss drugs became one of the hottest topics in health in 2023. Originally intended to manage diabetes, the most popular drug, semaglutide, has garnered massive popularity for its ability to promote quick weight loss in patients.
Semaglutide is the generic name for Wegovy, which is approved for weight loss, and Ozempic, which is used to treat diabetes. Both are made by Novo Nordisk.
Ozempic works by mimicking the hormone GLP-1, which helps the pancreas release insulin. It promotes weight loss by sending signals to the appetite center in your brain to reduce hunger and increase fullness, making it easier to manage hunger cues, stick to smaller and fewer meals and overcome emotional or boredom eating.
Semaglutide has been shown to help patients lose 15% to 20% of their body weight but is also costly and often not covered by insurance if used primarily for weight-loss purposes.
Costing around $1,000 a month for weekly injections, the drugs have also been shown to present some serious downsides, one of which is the tendency for consumers to put the weight back on after stopping treatment. There is also a low risk of pancreatitis, gallstone disease and gastroparesis.
Ozempic shortages
Semaglutide has been in short supply for about two years, largely because it has been increasingly prescribed off-label to people who don't have diabetes.
While Ozempic was approved for the treatment of diabetes back in 2017, Wegovy was only approved for use in weight loss in 2021, causing an explosion in demand.
As previously reported by USA TODAY, Novo Nordisk has struggled to keep up with the huge demand, as data released in September showed that prescriptions skyrocketed in major metropolitan areas within the first year of Wegovy's approval. In some locations, such as Cleveland and Seattle, prescriptions for semaglutide went up 481% and 351%, respectively.
This article originally appeared on USA TODAY: Counterfeit Ozempic warning issued after FDA seizes thousands of units

