First at-home combination test for COVID and flu authorized by FDA
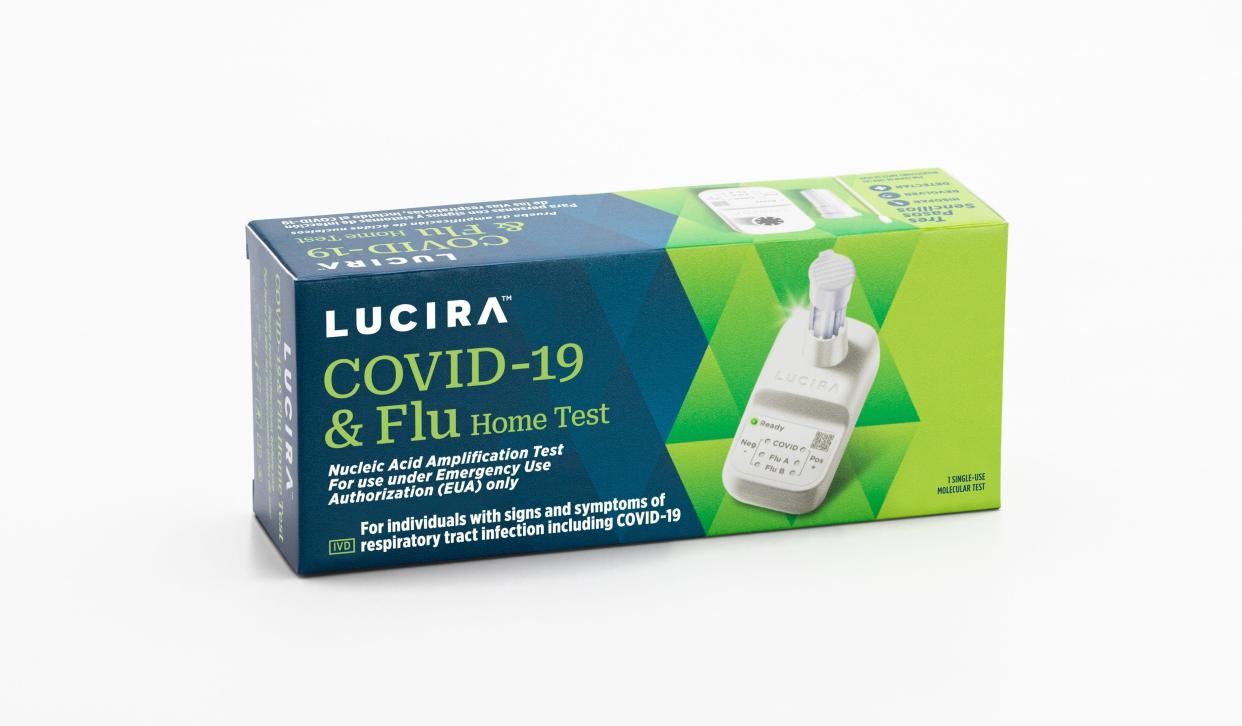
Now that we've all been trained on doing nose swabs for COVID-19 tests, we will soon have the option to test for the flu at the same time, too.
The Food and Drug Administration has authorized the first over-the-counter at-home test that can detect and differentiate between a test result for flu and a test result for COVID-19.
The Lucira COVID-19 and Flu Home Test is a single-use test, which can be purchased without a prescription. A nasal swab is used as with an at-home COVID test; in 30 minutes or less, the test displays the results – positive or negative for influenza A, Influenza B and COVID-19.
“Today’s authorization of the first OTC test that can detect Influenza A and B, along with SARS-CoV-2, is a major milestone in bringing greater consumer access to diagnostic tests that can be performed entirely at home,” said Dr. Jeff Shuren, director of the FDA’s Center for Devices and Radiological Health, in the announcement Friday.
Pay increases since 2010: Airline pilots got sharpest increases, dentists the least. Did your job make the cut?
'Strangest winter we’ve had yet': Southern California sees rare blizzard conditions, heavy rain
How much will an at-home COVID-19 and flu test cost?
Lucira Health began making its COVID-19 & Flu test available in Canada in August 2022 and it's currently priced at about $70.
Insurance coverage may also affect how much consumers pay.
Lucira Health will announce a U.S. price tag and release date at a later date, company president and CEO Erik Engelson told USA TODAY. "This is a major milestone for Lucira Health and at-home diagnostics, and I can’t thank our employees and partners enough for seeing this through, and of course, for the FDA’s recognition," he said.
The company currently also sells an Lucira Check It COVID-19 Test ($34.99), which is a molecular test – similar to polymerase chain reaction (PCR) lab tests – and considered more accurate than other at-home rapid antigen tests.
In addition to Canada, at-home tests for COVID and flu are already available in Australia and Europe (the test available there also tests for RSV), according to medical site WebMD.
Prescription rules: DEA proposes rules to limit telehealth prescriptions for Adderall, Ritalin and painkillers
Viruses: What you need to know about RSV, the flu and virus myths
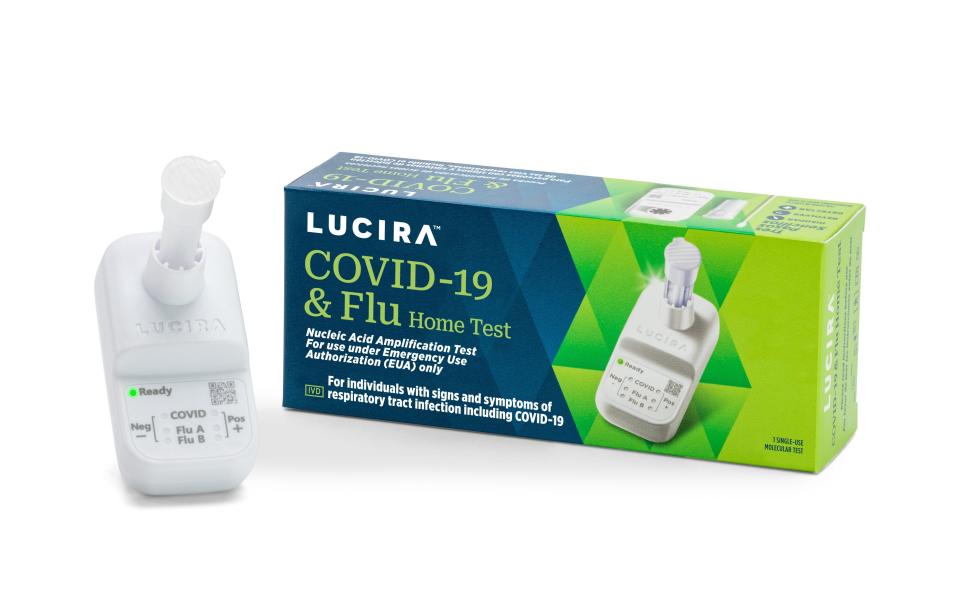
How accurate is the at-home COVID and flu test?
The FDA said the test correctly identified:
100% of negative and 88.3% of positive COVID-19 samples
99.3% of negative and 90.1% of positive Influenza A samples.
99.9% of negative Influenza B samples.
What is Lucira Health?
A medical technology company based in Emeryville, California, Lucira Health had hoped to receive an emergency use provision from the FDA at about the same time it got authorization from Canada.
It had already begun manufacturing tests to meet anticipated demand – as demand for its COVID-19 test declined. Without the expected revenue from the combo test, the company has filed for Chapter 11 bankruptcy protection and plans to pursue a sale of the business, it announced Feb. 22.
“Unfortunately, as restrictions lessened in 2022, we saw lower demand for COVID-19 tests. This, combined with slower than anticipated regulatory approval for the new combined test kit developed for the 2022-2023 flu season led to insufficient revenue and capitalization to offset expenditures," Engelson said in the bankruptcy announcement. "Despite every effort to reduce capital outlays and restructure our business, we took this action to protect and maximize the value of our assets.”
In January, the company announced the addition of a free Medi-Call telehealth call with the purchase of its COVID-19 and Flu test in Canada.
What's everyone talking about?: Sign up for our trending newsletter to get the latest news
Follow Mike Snider on Twitter: @mikesnider.
This article originally appeared on USA TODAY: First COVID and flu at-home test OK'd by Food and Drug Administration

