Like her mom, Wisconsin woman battled breast cancer. Now she's the first to complete vaccine trial.
Dr. Eva Vivian was a teenager when her mother, not yet 40, learned she had a breast tumor.
Vivian's memories aren't pleasant.
"The only option was a mastectomy. They were mean to women back then. It was a male-dominated profession," said Vivian, a professor in the University of Wisconsin-Madison's School of Pharmacy. "There wasn't a lot of empathy toward women who developed breast cancer."
Her mother lived 20 more years cancer-free before developing colorectal cancer. She died at age 62.
Months before her own 62nd birthday, Vivian developed the most aggressive form of breast cancer. It's known as "triple-negative" because the cancer cells lack three things that medical treatments normally can target. They lack hormone receptors for estrogen and progesterone, meaning hormone therapy is not effective. And they lack the protein HER2, meaning drugs targeting that protein do not work.
What's left as the main treatment options for patients with triple-negative breast cancer are chemotherapy and surgery.
Vivian underwent surgery, then three months of chemotherapy and additional radiation treatments, at which point she was declared cancer-free.
Given her experience, the experience of her mother, and her belief in preventive health care, Vivian volunteered to be one of more than 30 participants in a cancer vaccine trial underway involving UW-Madison's Carbone Cancer Center, Johns Hopkins University and the University of Washington School of Medicine.
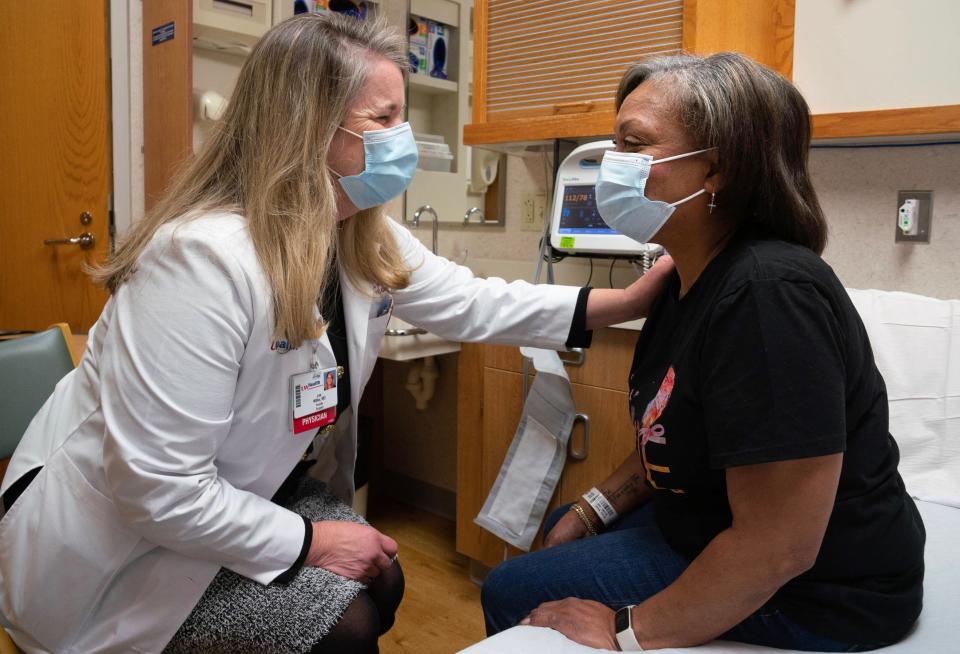
The schools are partnering on phase two of the clinical trial. This phase will determine how safe the vaccine is and how effective it is at preventing a recurrence of triple-negative cancer.
“We’re testing this vaccine to determine whether the patient’s immune system revs up to fight cancer cells and whether the cancer cells are there or not in patients with a previously treated triple-negative cancer,” said Dr. Lee Wilke, the senior medical director of clinical oncology services with UW Health and principal investigator for the clinical trial. She is also Vivian's oncologist.
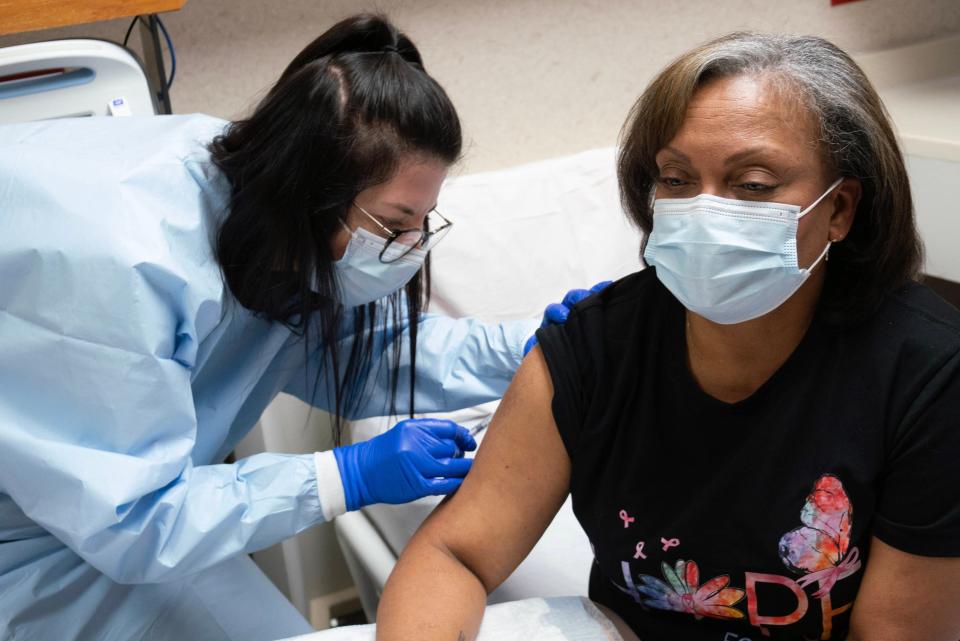
Vivian rolled up her sleeve for the third and final vaccine shot Jan. 18, becoming the first woman in the country to complete the three-shot trial to prevent triple-negative cells from again growing in her body.
“A vaccine that could prevent the development of breast cancer would be phenomenal because it means that women would not have to go through what we went through,” Vivian said, referring to her and her mother.
"Most women have friends or know someone who has gone through breast cancer treatment. And when you look at the challenges and the changes in lifestyle of a woman that goes through that type of cancer, having a vaccine is a lot easier than having cancer. I don't think you will see the type of vaccine hesitancy we saw with the COVID vaccine."
Vaccine research for cancer is in its infancy
Triple-negative accounts for 10% to 15% of all breast cancer cases, with those most susceptible being women under age 40, Black women and women who have a BRCA1 genetic mutation among the groups, according to the American Cancer Society.
If the DNA-based vaccine, developed by Dr. Nora Disis at the University of Washington-Seattle, is found to be effective, it would need to be approved by the Federal Drug Administration before being made available to patients. If that happened, it would be the first vaccine to treat any form of breast cancer.
While Wilke said many cancer vaccines are in the trial phases, there are only four approved for patient use by the FDA.
"Vaccine research for cancers is in its infancy," said Wilke, who is also a professor of surgery at the University of Wisconsin School of Medicine and Public Health. "This trial will give oncologists an understanding of how vaccines may help augment a patient’s immune system to combat cancer if it returns."
The trial, sponsored by the National Cancer Institute Division of Cancer Prevention, is still accepting patients. Researchers hope to enroll 30 participants. To qualify, participants must have had stages 1-3 of triple-negative breast cancer in the past three years, and been curatively treated.
Vivian other participants will receive a booster three months after the three-shot vaccine series, and a final booster six months after the first booster.
Regular mammograms are key, especially for high-risk groups
Because her mother had breast cancer when she was in her 30s, Vivian is considered high risk and has been receiving mammograms annually.
She said that saved her.
"I do feel, in general, providers need to be more vigilant about building good relationships with their African American patients," she said. "Providers need to build trust and actively encourage their patients to get mammograms on a regular basis. That is really vital."
In 2019, her mammogram discovered a small, three-centimeter tumor. While other forms of cancer might not have required chemotherapy to treat such a small tumor, the aggressive nature of triple-negative cancer made chemotherapy Vivian's only option.
Vivian said she experienced flu-like symptoms after the first two shots and was symptom-free following the third.
"Receiving a vaccine is like eating a piece of candy compared to chemotherapy," Vivian said.
Vivian, is a proponent of preventative medicine, with her own research including how to prevent diabetes, particularly in the Black community. In Milwaukee, she is teaching diabetes prevention to African American families at McGovern Park Senior Center and the World Outreach Center.
"African American women tend to have poor outcomes because they are diagnosed later. They are not getting mammograms early enough and often enough," Vivian said. "Until vaccines exist, early detection through mammograms remains key."
Those who meet the criteria and are interested in participating in the trial vaccine study can obtain more information or enroll at the trials.gov website or through UW Health.
Jessica Van Egeren is the Milwaukee Journal Sentinel's enterprise health reporter. She can be reached at jvanegeren@gannett.com.
This article originally appeared on USA TODAY: Wisconsin woman completes vaccine trial for aggressive breast cancer

