Kansas Citians with sickle cell disease may benefit from new FDA-approved treatments
Sickle cell disease affects about 100,000 people in the United States, including an estimated 2,000 in Missouri and about 700 Kansans.
On Friday, two new treatments for the blood disorder were approved by the U.S. Food and Drug Administration.
The treatments — Casgevy and Lyfgenia — are the first cell-based gene therapies for the treatment of sickle cell disease. Casgevy is the first FDA-approved treatment to use the CRISPR gene-editing technology to modify red blood cells, according to the FDA.
With the news on Friday, what does it mean for Kansas Citians living with the disease?
When can they receive these treatments? How does the treatment work?
The Star spoke to Kevin Wake, president of the Uriel E. Owens Sickle Cell Disease Association of the Midwest, who lives with sickle cell, and Donna McCurry, a senior nurse practitioner who works with the Sickle Cell Center at University Health (formerly Truman Medical Center) in Kansas City, for information regarding the newest treatments for the disease.
What would the new sickle cell treatment mean for people in Kansas City?
For many people living with sickle cell, either Casgevy or Lyfgenia would offer a new therapy that could cure many of the life-threatening impacts from the disease, Wake said.
“Patients would be able to be symptom- and pain-free and have a greatly improved quality of life that would allow patients to go to school, have careers and live life fairly uninterrupted from their disease,” he said.
When and where would people get the new treatment locally?
Wake and McCurry were not sure of the timeline to start treating patients with the new technology, but Wake said he could see it becoming available during the first quarter of 2024.
Treatment centers have not been released, but Wake said that based on past trial sites, the closest site for Kansas Citians would be Washington University in St. Louis. The Heartland Southwest Sickle Cell Disease Network is based out of Washington University’s School of Medicine and collaborates with hospitals in Arkansas, Iowa, Kansas, Louisiana, Missouri, Nebraska, Oklahoma and Texas, including University Health.

University Health treats around 105 adult patients for sickle cell, McCurry said. The oldest patient is 70 years old, and they have several that are over 50.
How do the treatments work?
Both Casgevy and Lyfgenia use a person’s blood product to prevent the sickling of blood cells, according to McCurry.
Casgevy involves the collection of stem cells, which are sent to a lab where genetic modification occurs to correct the DNA structure so that the blood cells no longer form a sickle shape.
Before the stem cells are infused back into the patient, they must undergo chemotherapy to clear remaining stem cells that could make sickle-shaped cells.

In the case of Lyfgenia, a person’s blood cells are modified with “gene-therapy-derived hemoglobin that functions similarly to hemoglobin A, which is the normal adult hemoglobin produced in persons not affected by sickle cell disease,” according to the FDA.
Red blood cells containing the modified hemoglobin have a lower risk of sickling and impeding blood flow. These modified stem cells are then delivered to the patient.
“Now we actually have tools to push back on those symptoms we see in sickle cell and can control it,” McCurry said. “The concept of control in sickle cell now was unheard of when I started back in 2008.”
A concern with the treatment involves the length of the process.
National news organization NPR health correspondent Rob Stein said, “The procedures are long, difficult and complex, requiring multiple trips to a hospital for testing, a grueling and potentially dangerous bone marrow transplant and lengthy hospitalization.”
Both treatments are intended for patients ages 12 and older.
What is sickle cell disease?
Sickle cell disease is a genetic blood disorder.
The disease causes a person’s red blood cells to be shaped like a sickle. Because of this, the cells do not carry the correct amount of oxygen throughout the body and restrict the flow in blood vessels, preventing oxygen from being delivered to organs and tissues in the body.
The clogging of cells can cause numerous side effects, including painful episodes that can last for hours, days and in extreme cases, weeks, according to Wake.
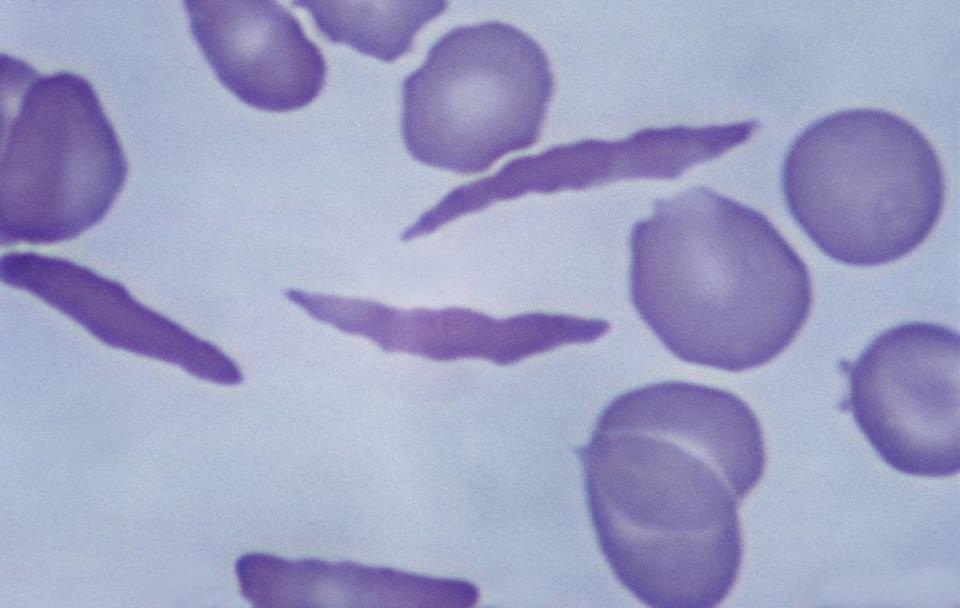
“Patients commonly have strokes throughout their lives, organ damage, and can have negative impacts wherever blood flows in the body, which is everywhere,” Wake said.
The disease is most common in Black people, according to the FDA. While less prevalent, the FDA said it also affects Hispanics.
How is sickle cell treated?
Before Friday’s approval of Casgevy and Lyfgenia, there were four FDA-approved treatments:
Hydroxyurea, an oral medication that has been shown to reduce or prevent several complications of sickle cell disease.
Oxbryta, an oral medication that prevents red blood cells from forming the sickle shape and binding together. The medication may reduce the destruction of some red blood cells, which can lower the risk for anemia and improve blood flow.
Adakveo, medication given through an intravenous line in the vein. It helps prevent blood cells from sticking to blood vessel walls and causing blood flow blockage, inflammation and pain crises.
L-glutamine, an oral medication that helps lower the number of pain crises.
Side effects for these treatments include headache, diarrhea, abdominal pain, nausea, fatigue, fever, joint pain, back pain, a lowered white blood cell count or platelet count
Besides these FDA-approved treatments, Wake said that blood transfusions are common for treating the disease. Bone marrow transplants can cure the disease but are difficult because of the lack of donor matches and the need for taking lifelong anti-rejection medication after the transplant, according to Wake.
How do people get sickle cell disease?
People are born with the disease. It is inherited when a child receives two genes — one from each parent — that have abnormal hemoglobin.
Having the sickle cell trait typically causes few symptoms for individuals.
How do you know if you have the sickle cell trait? McCurry said you your blood can be tested for the form of hemoglobin that carries the trait.