Ken Baker: Scientific community may have the "Ring of Power" to end malaria
Take it Gandalf! Take it!
No, Frodo.
You must take it!
You cannot offer me this Ring!
I'm giving it to you!
Don't tempt me Frodo! I dare not take it. Not even to keep it safe. Understand Frodo, I would use this Ring from a desire to do good. But through me, it would wield a power too great and terrible to imagine.

Suppose Sauron — or perhaps Gandalf — were to offer you a magical golden ring with the power to rid the world of mosquitoes once and for all. And with their demise, to save countless human lives from the many diseases for which mosquitoes are the vectors. Malaria alone kills more than 600,000 people each year, mostly under the age of 5.
More: Baker: New tools can change mosquitoes' DNA, but should it be done?
That ring — scientific technology — exists and is now being refined and tested in the Elvin forges of academic, commercial, and government research facilities around the world. In this essay let’s refer to it by the ungainly name, CRISPR-Cas/Genome editing/Gene drive procedures.
In my last essay, I briefly discussed how the CRISPR (DNA)-Cas (protein) system found in many bacteria (and archaea) defends its cell against attack by viruses, and that scientists have learned how to modify the system into a tool to find and cut almost any gene found in many types of animals, plants and fungi.
Why would they want to do that? Having cut the gene at a desired location, they can then use various other molecular tools (in a process called genome editing) to add or remove bits of DNA to the gene that will change how it works.
Genetic change could spread through an entire species
But it’s one thing to drop a piece of DNA that would, say, cause a given mosquito to produce sterile female offspring, and another to have that genetically based trait spread throughout an entire population of mosquitoes. That’s where “gene drives” come into play.
OK, let’s stop here for a refreshing breath of honesty. There’s no way I can adequately explain how gene drive procedures work without delving into a lot of molecular biology. Nevertheless, I still want to offer a bit of an introduction, so here goes…
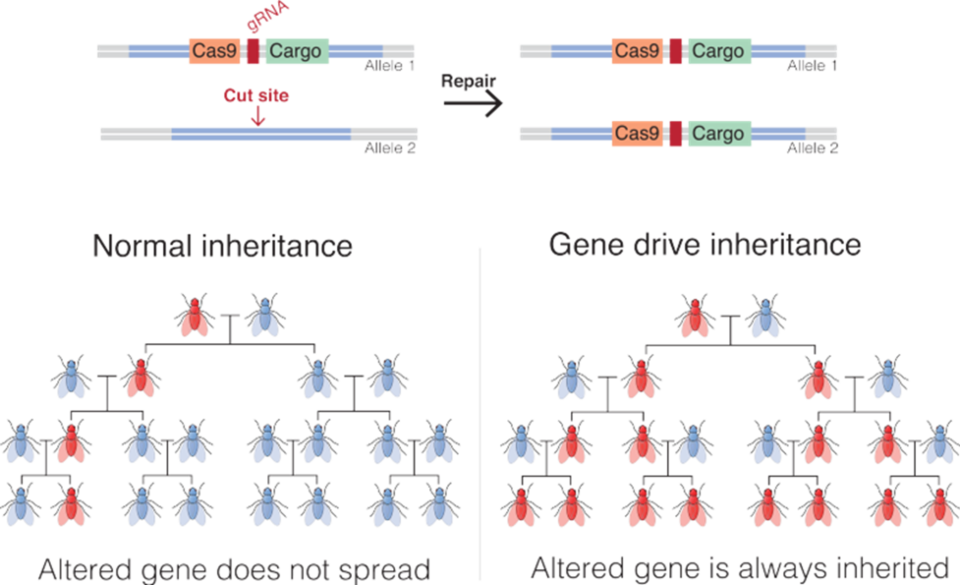
If, when our researcher adds a sterility-causing piece of DNA to a particular gene in a mosquito, she also inserts a second piece of DNA coding for (leading to the production of) the several parts of a CRISPR-Cas system, then…
Well, then, something remarkable takes place. The CRISPR-Cas system will use a copy of the sterility-causing gene to turn any normal (non-sterility) gene in that insect into the mutant form. Thereafter, all the babies of that mosquito will not only get the mutant gene but also the DNA coding for CRISPR-Cas … and that mutant gene will then spread to the all the offspring of the mosquitoes those babies eventually mate with.
In just a few generations, every female mosquito in the population will be sterile and the population will go extinct. Is this something we would want to do?
What is meant by the word “we” in the last sentence?
Over the past decade, numerous workshops and conferences have focused on the ecological, regulatory, social, and ethical ramifications of the critical question of whether gene drive technology should be used in the natural world.
Scientific community examining the issues
However, the overwhelming number of voices debating questions on if, when, and where the first gene-drive based attacks on malaria should occur are those of researchers and ethicists from the high-income countries where gene drive technologies were developed, without equal input from those who live in the low-income countries under discussion.
To be fair, the scientific community is well-aware of the problem and there’s a lot of talk on how to include local stakeholders in all stages of the development and possible deployment of the technology. But how do you accomplish this when the gap in knowledge between the scientists who understand the technology and villagers who would be directly impacted is so great?
Even the very notion of involving “local” stakeholders in such discussions is problematic. Gene driven changes within a population are designed to spread, and mosquitoes don’t pay attention to borders between one village or country and the next. As pioneer gene drive researcher Keven Esvelt has put it, “a release anywhere, is likely a release everywhere.”
And there are so many unknowns and what-ifs. Critics rightly point to the very real potential for unanticipated ecological impacts. What if a gene driven effort to control a pest insect has problematic (for us) side-effects on the species or, worse, spreads to non-target species through interbreeding?
Yes, but children are dying.
In the "Lord of the Rings" novels, the ring of power was clearly evil and had to be destroyed. But in this world, the use of our ring of power — knowledge — will always carry risks of harm along with potential benefits. How are we to decide if and when the benefits outweigh the risks?
What do we mean by the word, “we”?
Ken Baker is a retired professor of biology and environmental studies. If you have a natural history topic you would like Dr. Baker to consider for an upcoming column, please email your idea to fre-newsdesk@gannett.com.
This article originally appeared on Fremont News-Messenger: Baker: Genetic splicing could eliminate mosquitoes, but at what cost?

