Lilly (LLY) Gets FDA Nod for Expanded Use of COVID Cocktail
- Oops!Something went wrong.Please try again later.
Eli Lilly & Company LLY announced that the FDA has expanded the Emergency Use Authorization (EUA) for its cocktail antibody medicine, bamlanivimab plus etesevimab to include the post-exposure prevention (prophylaxis) for COVID-19 indication.
This means the cocktail medicine can now be used (on an emergency basis) to treat certain people who have been exposed to the virus or who are at high risk of exposure in an institutional setting, including a nursing home or prison. The expanded authorization is based on data fromtheBLAZE-2 study.
Please note that bamlanivimab (700 mg) plus etesevimab (1400 mg) together is presently approved for emergency use to treat mild-to-moderate COVID-19 in high-risk patients. Neither of the antibody drugs is approved for monotherapy use.
Data from the BLAZE-2 study showed that bamlanivimab significantly reduced the risk of contracting symptomatic COVID-19 by up to 80% in nursing home residents and up to 57% among residents and staff of long-term care facilities.
Lilly’s shares have risen 36.8% this year so far compared with the industry’s increase of 9.4%.
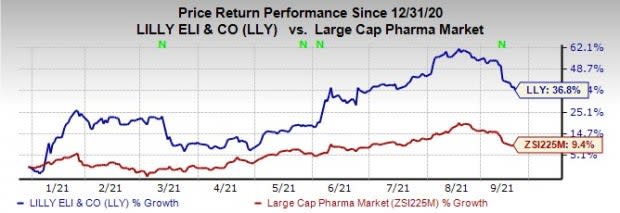
Image Source: Zacks Investment Research
The rising infection rates in the United States, mainly due to the spread of the highly contagious Delta variant, have now increased the demand for antibody drugs like Lilly’s cocktail medicine. Particularly, nursing home residents and individuals with medical conditions are at high risk of the most severe outcomes. Despite being fully vaccinated, there are an increasing number of cases of nursing home staff getting infected. The approval of Lilly’s cocktail antibody medicines for post-exposure prophylaxis should help prevent further spread of the infection in this high-risk population.
Please note that sales of Lilly’s COVID medicines have been below the company’s expectations in the first half of 2021 due to lower demand amid rising vaccinations and increased competition.
We remind investors that bamlanivimab was the first neutralizing monoclonal antibody to be granted EUA by the FDA for treating mild-to-moderate COVID-19. However, bamlanivimab’s EUA as a monotherapy treatment for COVID was revoked by the FDA in April at Lilly’s request. Lilly decided to focus only on supplying its antibody cocktail, bamlanivimab and etesevimab together, which was granted emergency approval by the FDA in February.
The latest approval of bamlanivimab plus etesevimab combination for post-exposure prophylaxis may increase demand for the medicines and result in better sales in the second half of 2021.
Another antibody cocktail medicine, Regeneron Pharmaceuticals REGN, REGEN-COV (casirivimab and imdevimab) is already approved (on an emergency basis) for post-exposure prophylaxis in people at high risk of progression to severe COVID-19 apart from being approved for treating certain infected patients to reduce the risk of hospitalization or death from COVID-19.
Another monoclonal antibody granted EUA by the FDA is GlaxoSmithKline GSK and Vir Biotechnology’s VIR sotrovimab for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients.
Lilly currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Vir Biotechnology, Inc. (VIR) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
