Take a look at your nasal spray — there’s a recall for possible microbial contamination
All bottles of three nasal rinse sprays sold over the last six years by Biomic Sciences have been recalled after FDA testing found four kinds of microbial contamination.
In its recall notice, Biomic says customers should stop using Ion Biome Sinus, Ion Sinus Support and Restore Sinus Spray. The recall notice’s risk statement explains:
“In the population most at risk, patients or individuals who recently underwent nasal or sinus surgery, there is a reasonable probability that the use of the affected product could potentially result in severe or life-threatening adverse events such as bacteremia or fungemia, invasive bacterial or fungal rhinosinusitis, or disseminated fungal infection.”
READ MORE: Medication used to treat yeast infections might have contamination
The sprays are sold in stores and by various online sellers.
▪ Restore was sold from June 2017 to September 2019.
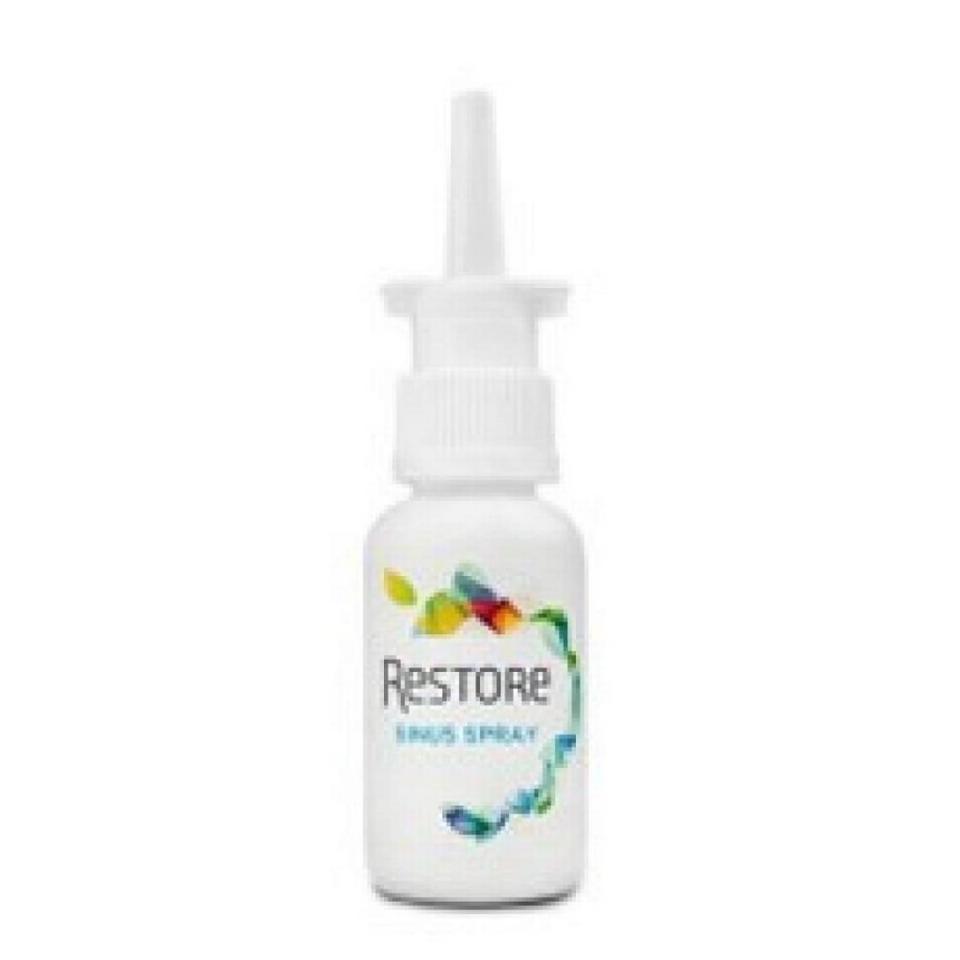
▪ Ion Biome Sinus was sold from September 2019 through September 2021.

▪ Ion Sinus Support was sold from September 2021 through last month.
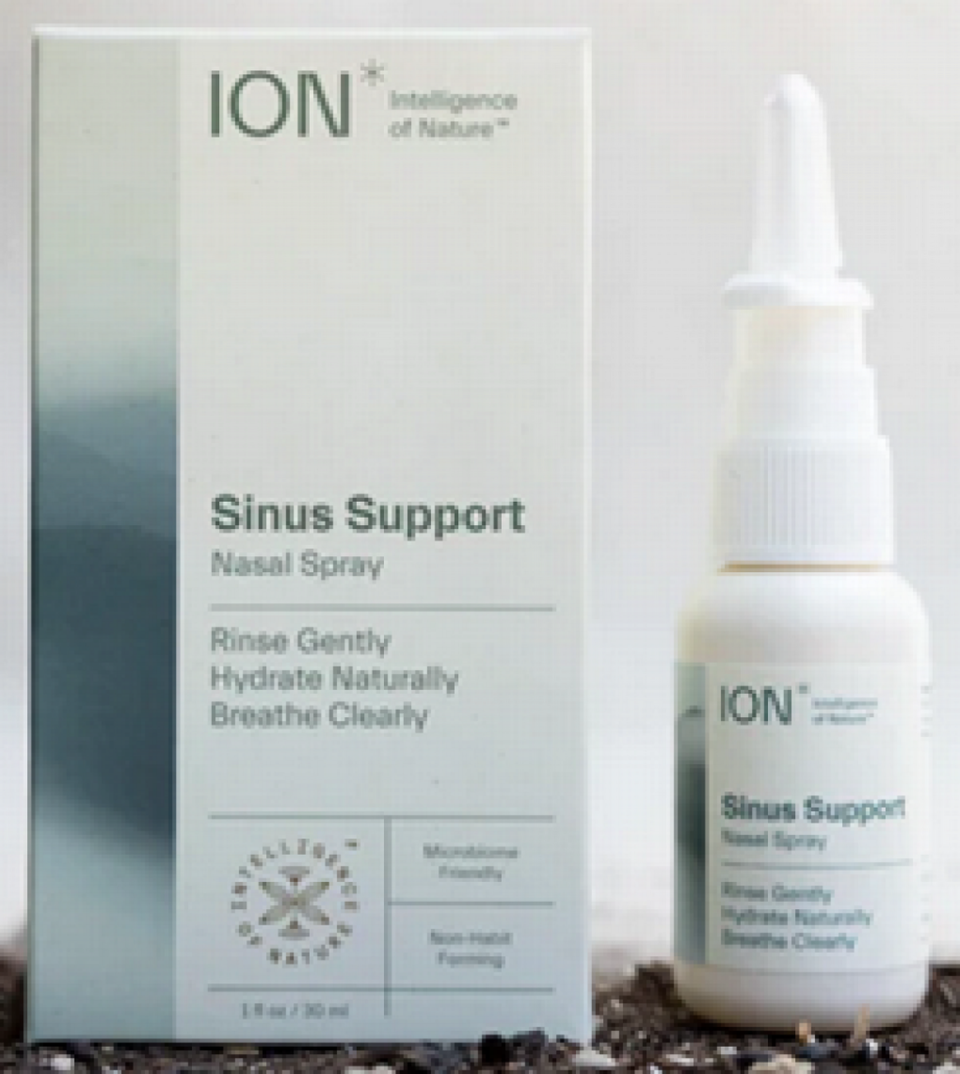
If you have one of these bottles and want a refund, take a photo of the lot number on the bottle bottom and send the photo to Biomic at sinusrecall@intelligenceofnature.com. Or just return it to the store where you bought it.
Questions about the recall can be answered at the above email address or 844-715-0113, Monday through Friday, 9 a.m. to 5 p.m., Eastern time.
Any medical problems having to do with these sprays should be taken to a doctor. Then, let the FDA know about it through the MedWatch program, either online or by mailing or faxing the FDA a form you request at 800-332-1088.

