Making history: Massillon boy among first to receive new treatment for Duchenne
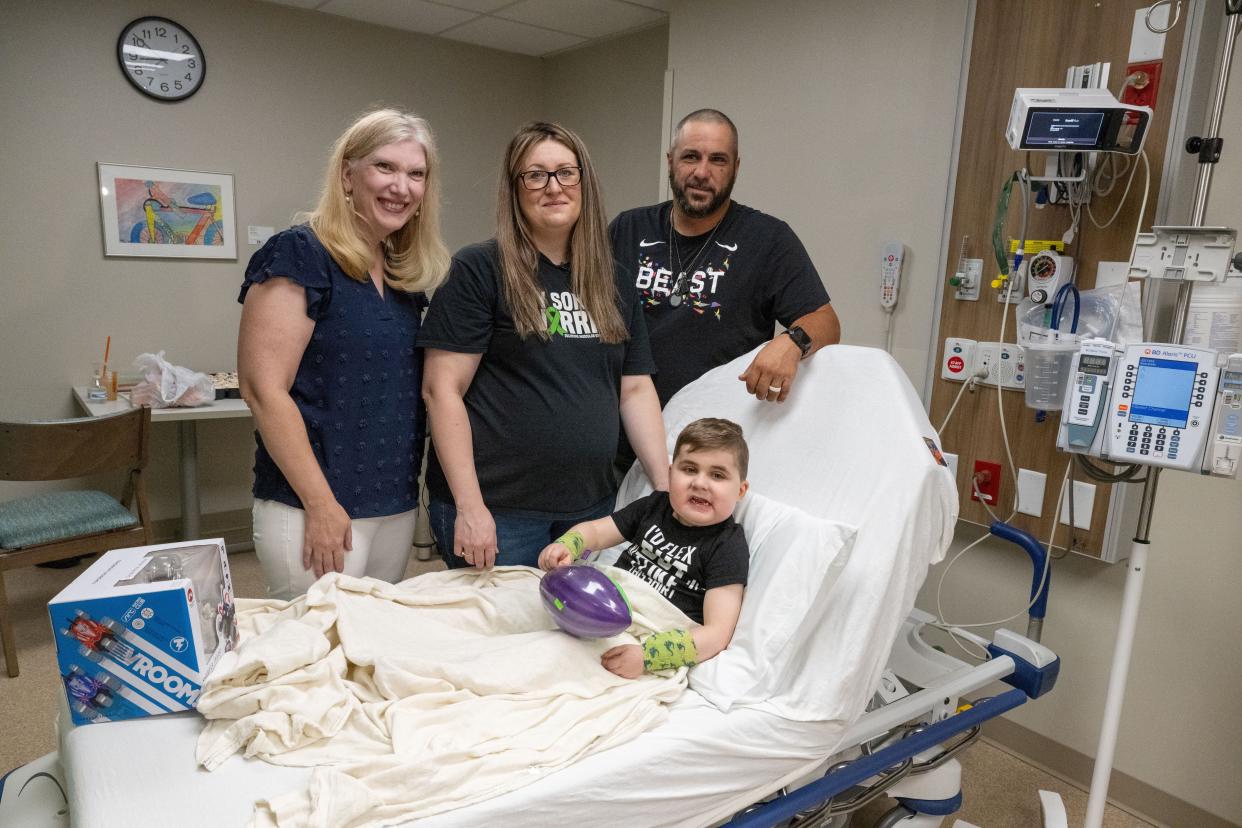
After years of medical visits and unanswered questions, Jill DeMando was frustrated and confused.
Her son Vinny was struggling physically to keep up with children his age. At 3 years old, he frequently stumbled while walking or climbing up stairs. He could not run and jump with his friends.
The doctors said everything was OK, but the Massillon mom suspected that there was more to the story.
She was right. In 2021, 3-year-old Vinny was diagnosed with a fatal neuromuscular disease, Duchenne muscular dystrophy (DMD). Two years after the devastating diagnosis, there was a glimmer of hope. After promising clinical trials, the Food and Drug Administration approved a new DMD treatment, Elevidys.
Last week, 6-year-old Vinny and another patient in Columbus became the first Ohioans to receive the treatment after the federal approval. Vinny received his treatment at Akron Children's Hospital.
What is Duchenne muscular dystrophy?
Duchenne muscular dystrophy is a rapidly progressing disease that attacks the muscles.
Dr. Kathyrn Mosher, Vinny’s pediatric physical medicine and rehabilitation specialist at Akron Children's, said DMD is caused by a mutation in the gene, dystrophin.
Dystrophin is responsible for protein production. A lack of dystrophin leaves muscles fragile, breaking down easily with normal activity.
DMD appears predominately in boys, affecting 1 in 3,600 male infants. Symptoms begin to appear from ages 2 to 6. Most individuals do not survive past their 20s.
There is no cure for DMD. Current treatments aim to manage the disease and slow progression.
Akron Children's Hospital: New regional behavioral health center opens in Canton
Vinny’s diagnosis: 'It was very difficult to handle'
Vinny’s symptoms began to appear the moment he was born.
At 3 weeks old, his newborn screening bloodwork showed elevated creatine kinase liver enzyme levels. This elevation comes in conjunction with muscle weakness and damage.
Jill and Michael DeMando, who works at Southway Fence, have another child, 8-year-old Eliana. DeMando began noticing the differences between her daughter and son quickly – Vinny was exhibiting signs of developmental delay.

He was unable to crawl until he was a year old. He did not walk until 15 months and has never been able to jump.
After years of being told Vinny was a perfectly healthy child, DeMando finally asked for a referral to a specialist.
That is when the DeMando family met Mosher at Akron Children’s hospital. Almost immediately after meeting Vinny, Mosher was able to identify a diagnosis.

“They were going to do genetic tests and bloodwork, but she could tell us with pretty much certainty that it was Duchenne,” said DeMando, 37.
Vinny frequently did a Gower’s maneuver, a movement where a patient uses their hands and arms to walk up from a squatting position due to lack of muscle strength — a clear sign of DMD.
“After three years of concern and being told that your baby is fine – it was very difficult to handle,” DeMando said.
Since his diagnosis, Vinny has been taking steroid treatments, attending physical therapy, occupational therapy and numerous other treatments.
A new treatment backed by hope: Elevidys
On June 22, the FDA approved Elevidys injections as a treatment for DMD – a form of gene replacement therapy. The therapy was part of the FDA’s accelerated approval pathway, a program that aims to rapidly approve life-changing treatments for fatal conditions.
"It was designed to allow products to come to patients sooner who have life-threatening illnesses,” said Mosher. “For these patients, the risk of accepting a therapy that we don’t have much long-term data on is, in a certain sense, a risk they are willing to take.”
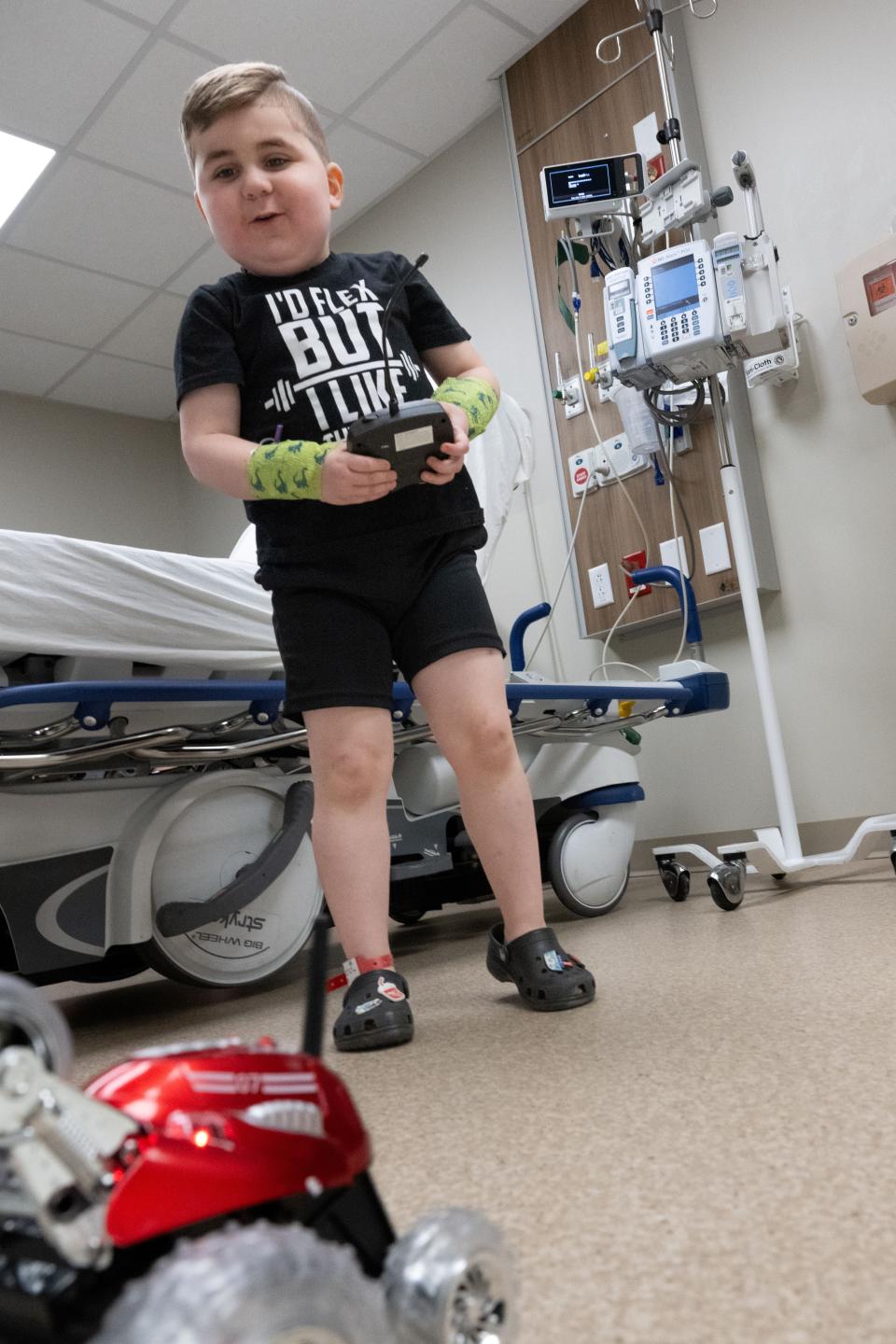
The treatment is a one-time IV infusion, lasting one to two hours. After the infusion, the patient is observed for several hours to monitor side effects.
For several months after, weekly bloodwork and an increased dose of steroids are required to monitor and suppress symptoms.
“The hope would be for him to have the energy and endurance level like your typical 6-year-old would have and be able to keep up with his peers,” DeMando said.
The treatment works to strengthen muscles and ease progression of DMD.
“Hopefully Vinny will be able to walk for a longer period of time and be able to participate in age appropriate activities in a way that he previously would not have been able to,” said Mosher.
Perfect timing: 'The system definitely worked the way it was supposed to.'
The infusions are FDA-approved for patients aged 4 and 5 years old.
For Vinny, the timeline was what many would consider a miracle.
He received his treatment on Aug. 4, just nine days before his sixth birthday on Aug. 13.

“The system definitely worked the way it was supposed to in this case," said DeMando, an account manager at Synchrony Financial. "I am very thankful for that."
Within a week of beginning the approval process, Akron Children's Hospital was able to begin ordering the drug and preparing for Vinny's treatment.
“As an institution, we worked very hard to do everything we possibly could to make sure that he did not miss out on the opportunity to receive this before his birthday," said Mosher.
Luckily, everything fell into place for Vinny. He successfully received his treatment before aging out of eligibility.
Testing is being done to allow for a wider range of ages to receive Elevidys. Mosher predicts more clinical data will be delivered to the FDA by January.
More: After unexpected heart diagnosis, Armonnie Hawkins' family urges vigilance
Mosher: A treatment, not a cure
While Mosher is hopeful, she feels it is important to mention that this infusion is a treatment, not a cure.
"There will be limits to the benefits that patients have," said Mosher. "They will likely end up needing to have additional types of treatment to support their well-being.”
And although Elevidys isn't putting an end to DMD, it is providing patients with hope. Vinny is the first of many to receive the potentially life-changing treatment.
"It’s a big deal to get to see it happen in real life and to be able to finally have something that seems effective to be able to offer to our patients," said Mosher.
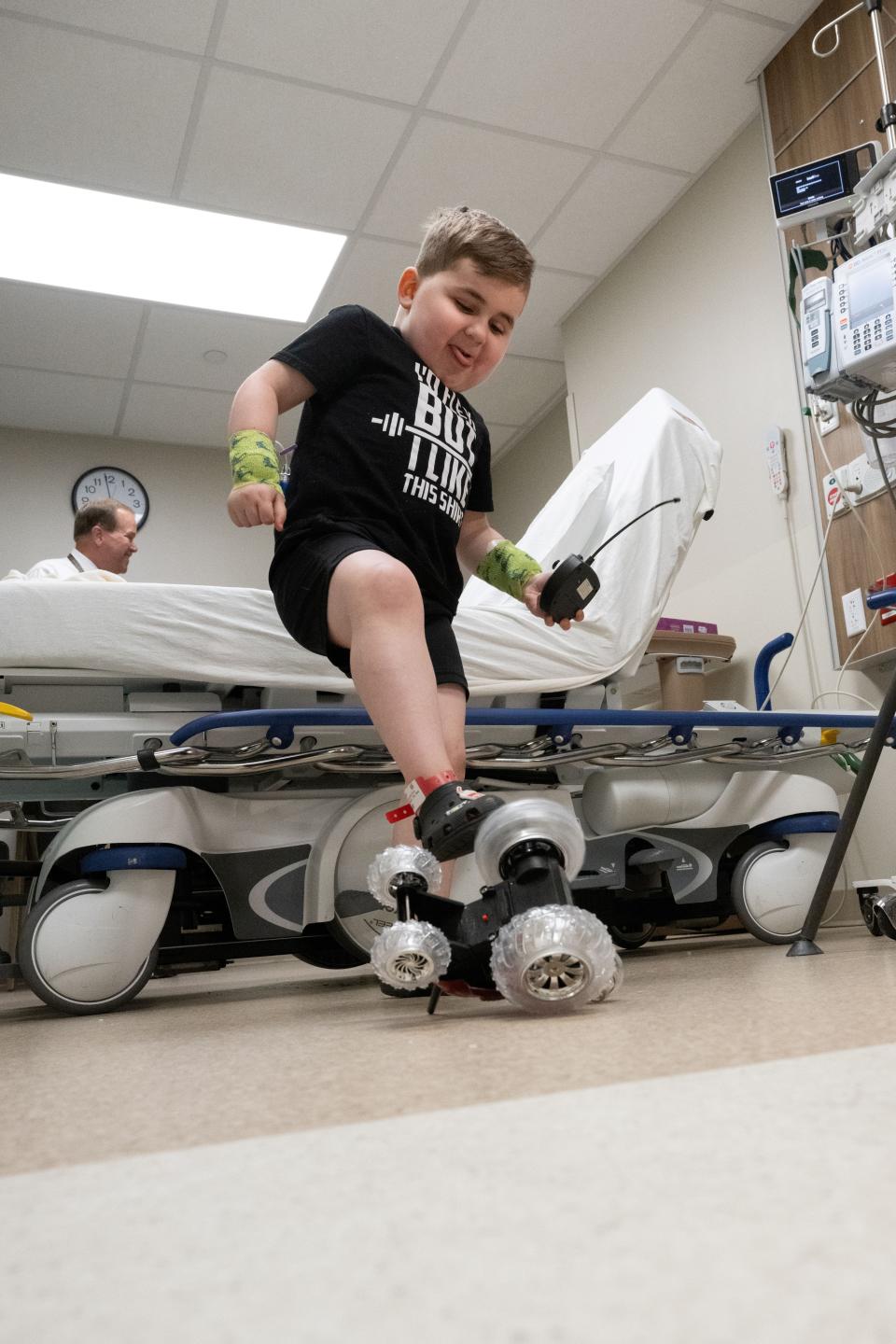
As the family prepared to celebrate Vinny's sixth birthday, DeMando noted the importance of being thankful. To her, the treatment is a step in the right direction.
"I think it teaches you a lot about not taking things for granted and just living the way that you're supposed to," she said. “To have it slowed in any capacity, for me is the biggest thing."
Contact Abreanna Blose by email at ablose@gannett.com or by phone at 330-580-8513.
This article originally appeared on The Repository: Massillon boy one of first to receive new muscular dystrophy treatment

