Miami kids needed surgery after infections set in. Were contaminated needles to blame?
When a 4-year-old boy came out of surgery at Nicklaus Children’s Hospital, the doctor cautioned his father that “his arm was going to look like he got bitten by a shark.”
The boy underwent surgery in January 2022 to remove a festering bacterial infection in his arm and thigh. He’s among 13 children, from newborns to teenagers, who were treated at Nicklaus within a span of months for mycobacterium abscessus, according to a lawsuit filed by the children’s parents.
The bacteria — which is found in soil, dust, and water and can cause skin infections through contaminated needle injections — is resistant to antibiotics and requires surgery to remove abscesses.
All had visited their Kendall pediatrician, Dr. Esther Marin-Casariego, for routine childhood immunizations during the spring and summer of 2021 shortly before the infections developed, states the lawsuit filed last year in Miami-Dade Circuit Court. All were vaccinated with “defective” syringes made in China that were supplied by McKesson Medical-Surgical, a division of the pharmaceutical giant, McKesson, according to the suit.
“We believe the common denominators are the needles,” said Miami attorneys Brett and Judd Rosen, adding that the vaccines were ruled out as the cause because the children received different vaccines, brands and formulations.
“McKesson is blaming the doctor,” they said.
And the doctor is blaming McKesson, because it supplied the needles she used to vaccinate her patients. Her lawyer said it’s the “only common factor” to explain the contamination cluster in her office.
The Goldberg and Rosen law firm is representing at least 13 families, including lead plaintiff Sophia Linale, 20, in a court battle against the pediatrician and McKesson, one of the largest pharmaceutical companies in the U.S., whose revenues were $276.7 billion in the fiscal year ended March 31, up 5% over the previous year, according to the company. (McKesson in 2022 agreed to pay $7.4 billion in damages for its role in the fentanyl-opioid scandal.)
The families’ lawyers estimate that as many as 100 children treated by Marin-Casariego may have been infected by the McKesson-supplied syringes.
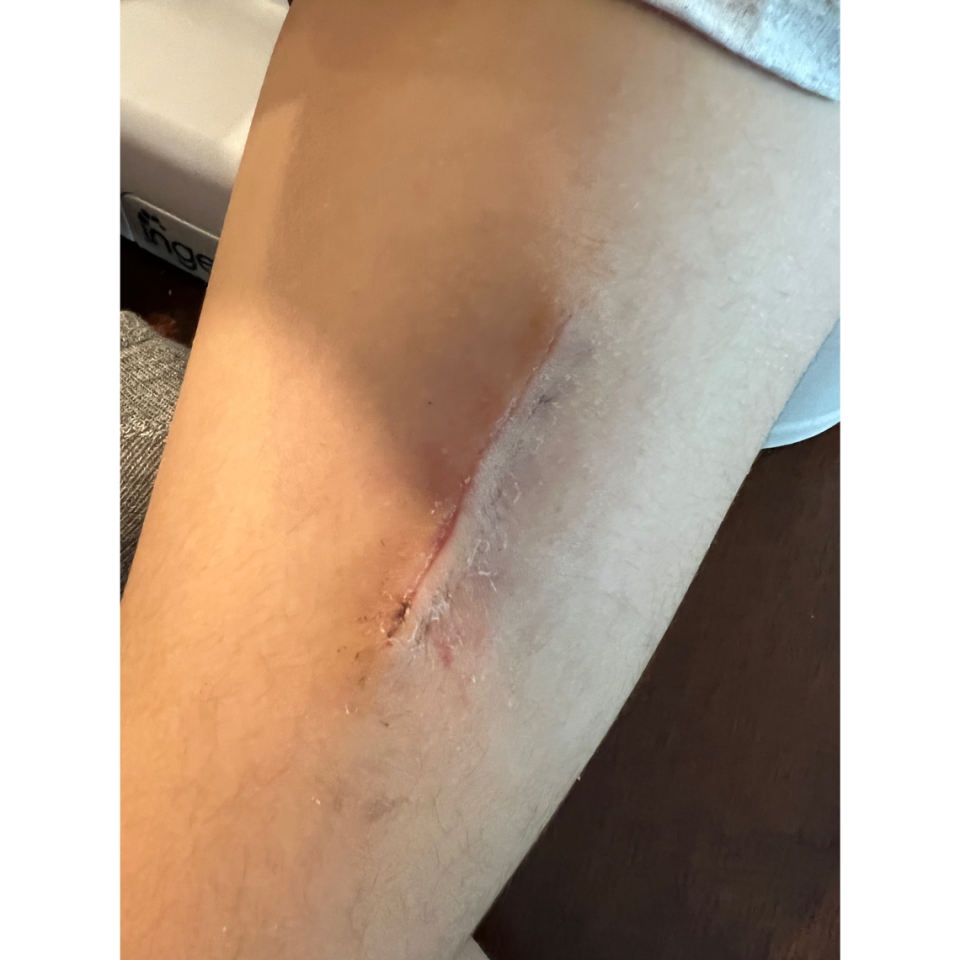
Linale, who has an underlying heart condition, was vaccinated in the summer of 2021 at 18. Shortly after vaccination, the teen developed severe mycobacterium abscessus in the injection site and had to undergo a “dangerous surgery under general anesthesia to remove the abscess and infection from her body” in December 2021, according to the lawsuit.
‘Scared to death’
“We were scared to death because our daughter had a heart problem,” said Ricardo Linale, who lives with his wife, Lianne, in Islamorada and formerly in Cutler Bay.
“We had no idea what was going on until she had the surgery,” he said, adding that the removal of the abscess left a “huge scar” on her arm.
The suit brought against McKesson Medical-Surgical accuses the company of negligence and product liability for supplying “defective” Chinese-made syringes contaminated with bacteria that infected the patients of Marin-Casariego, who used them to administer vaccines during the spring and summer of 2021. The suit also accuses the pediatrician of medical malpractice..
“Dr. Marin-Casariego has admitted to affected parents and their families that the [bacterial] infection is the result of improperly sterilized and/or contaminated needles and/or syringes distributed by defendant, McKesson,” the suit states. The pediatrician “breached the standard of care when [she] injected [her patients] with needles and/or syringes that were infected with a dangerous bacterium without first ensuring that the equipment and her office were free of any contaminants.”
Marin-Casariego has filed a “cross claim” against McKesson, saying the company is to blame because it distributed the contaminated needles to her. The doctor’s online profile with the Florida Department of Health does not list any disciplinary actions and her license is listed as clear and active.
Marin-Casariego’s lawyer said she referred her affected patients to a Nicklaus Children’s Hospital surgeon to have the bacterial infections and resulting abscesses removed as soon as she discovered they did not respond to antibiotics.
McKesson says it is not responsible for the children’s bacterial infections and subsequent surgeries, blaming the Kendall doctor while asserting the families’ negligence claims against her “are based on her own conduct,” according to the company’s motion to dismiss Marin-Casariego’s cross claim against McKesson.
However, U.S. government records show the company did not alert federal regulators about the needle contamination issue until after it was sued in January 2022 — despite being warned by Marin-Casariego about McKesson’s potentially tainted syringes three months earlier.
In two Feb. 14, 2022, reports filed with the Food and Drug Administration’s MAUDE digital system, McKesson noted that Marin-Casariego spoke with a company representative before Oct. 12, 2021, indicating that “several of her patients had developed infections” after receiving vaccinations in her office within the last six months.
Then, via email on Oct. 12, 2021, the pediatric physician said “she believed that the McKesson labeled needles/syringes were the common factor and may be the reason for the development of these infections that resulted in abscesses.”
The pediatrician, according to the two reports, did not provide “any product lot information regarding the McKesson labeled needles/syringes nor any patient specific information regarding the adverse events.”
McKesson acknowledged in the reports to the FDA that Marin-Casariego received four different types of syringes from the pharmaceutical company, including two under the McKesson label and two from another supplier, Becton Dickinson. “As a result of the complaint” from the doctor, McKesson said it was alerting the FDA about the company’s labeled syringes.
Lawyers for the pediatrician’s patients said McKesson only reported the doctor’s complaint about its needles after it was sued, adding that the Becton Dickinson syringes were not considered part of the infection cluster.
When asked about the reports sent to the FDA by McKesson, FDA spokeswoman Audra Harrison, in an email to the Herald, said the agency works to “quickly assess and address potential risks to health associated with medical devices” — but that it does not discuss “compliance matters, except with the company involved.” The agency did not explain what steps it took to address the possible syringe contamination problem McKesson reported to the FDA.
Speaking generally, Harrison said the agency monitors the safety of medical devices and that manufacturers are required to submit medical device reports “when information reasonably suggests that their device may have caused or contributed to a death or serious injury, or it has malfunctioned and that device or a similar device they manufacture would be likely to cause or contribute to a death or serious injury if the malfunction were to recur.”
The FDA says health care professionals, consumers and patients can voluntarily submit reports of adverse events and device malfunctions through its MedWatch Online Voluntary Reporting Form.
Florida health department, CDC investigate
“There’s clearly a case of underreporting by the drug company,” Judd Rosen said. “They are seeking to cover up or sweep something under the rug.”
Both Brett and Judd Rosen, who are working on the case with Miami lawyer Mustafa Dandashly, said it’s possible that other doctors in Florida or around the country could have received this same batch of needles as Marin-Casariego did.
McKesson’s Miami lawyer, Spencer Silverglate, said his client is blameless. In a statement, Silverglate said the Florida Department of Health and the Centers for Disease Control and Prevention “conducted a thorough investigation of the matter and determined that the infections likely arose from a transitory contamination of the water supply to the doctor’s office.”
Silverglate further stated that the two agencies “found no issues with the McKesson-branded hypodermic needles themselves, millions of which were distributed across the United States. No other purchaser of McKesson-branded hypodermic needles reported an outbreak of infections.”
Silverglate provided the agencies’ summary, based on assessments and samples taken from the doctor’s office in January 2022. The summary noted that three strains of the infectious bacteria were found among the doctor’s patients, but no such strains were detected in her medical office from “environmental sampling.”
“Contamination of medical supplies from sink splashing likely contributed to transmission of [Mycobacterium] abscessus strains which [was] resolved after supplies were exchanged,” the summary reads.. It noted “sink hygiene gaps,” with the splashing affecting certain medical products, including refillable soap dispensers and skin preparation supplies.
The agencies’ inspectors noted there were nine confirmed infectious cases and 39 probable cases among the doctor’s pediatric patients. Of the confirmed cases, three distinct strains of abscessus were found — but “no [Mycobacterium] abscessus was isolated from environmental sampling” in the doctor’s office.
The families’ lawyers countered that McKesson’s assertions distorted the reality of the agencies’ investigation of Marin-Casariego’s medical office. The Rosens cast doubt about “transitory water contamination” infecting the McKesson syringes in the doctor’s office, saying the state and federal agencies took “various samples and tests” and “were unable to locate any mycobacterium abscessus in the office or on any of the equipment.”
Moreover, they pointed out that the state and federal inspectors “were unable to test the McKesson needles because they had been disposed of after use on patients.” The Rosens also said McKesson kept no records of where the syringes went after being imported from China — including whether part of the shipment sent to Marin-Casariego’s office was also distributed to other physicians in the United States.
The physician’s Miami lawyer, Kimberly Cook, echoed that interpretation.
“Dr. Casariego reported her concerns about the needles to both McKesson and the FDOH,” Cook said in a statement. “McKesson did nothing to investigate these unfortunate incidents and the state investigated without coming to any official conclusion — at least not one that they shared with Dr. Casariego.
“The [McKesson’s] comment about ‘transitory water contamination’ is total speculation without any scientific basis,” she added.
South Florida families affected
After Marin-Casariego vaccinated her patients and they developed their bacterial infections, she referred them to a surgical specialist at Nicklaus Children’s Hospital.
Nicklaus pediatric surgeon Felipe Pedroso indicated to the children’s parents that he “operated on somewhere between 50-100 patients of Dr. Marin-Casariego who developed the exact same infection following vaccination,” the families’ lawsuit states.

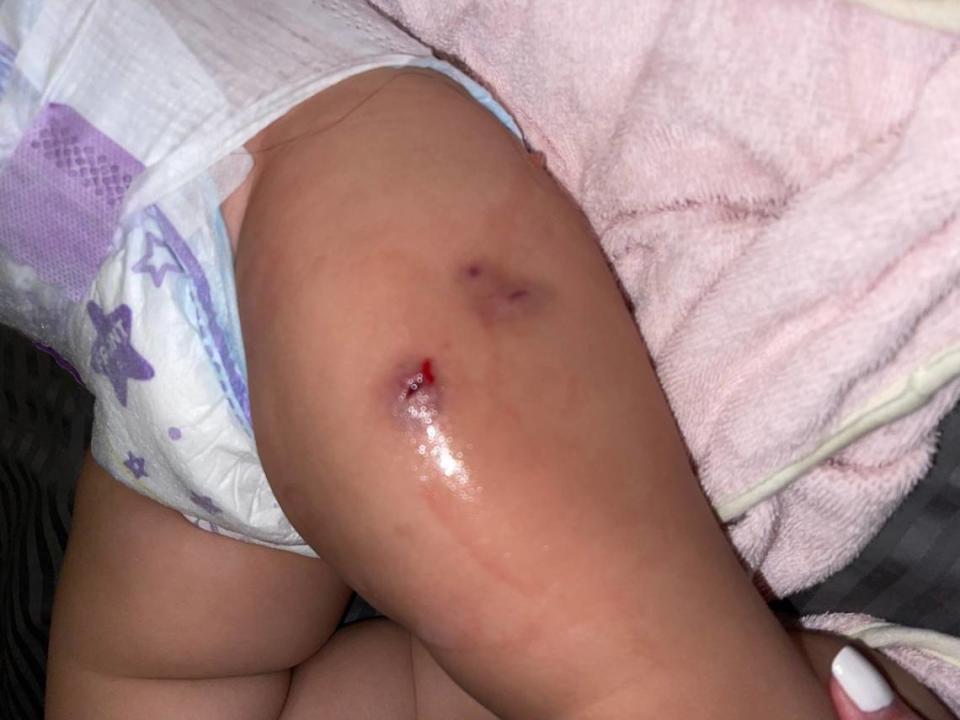
Arnold and Ailyn Cuervo knew something was wrong when they noticed their 1-year-old daughter, identified as A. C. in the suit, developed three warm, round red-purple quarter size lumps on her thighs following her vaccinations. At first, Marin-Casariego told them it was likely a reaction to the vaccines and that it would eventually go away. Months passed. And then finally, the lumps erupted, spewing a steady stream of pus.
Marin-Casariego helped secure an appointment with a Nicklaus specialist for the family. The baby had to be put under general anesthesia for three surgeries to clean out the infection and now has three large scars on her thighs, her parents say.
“She was injected with bacteria, it’s literally the opposite of what a vaccine is supposed to do,” said Ailyn Cuervo.
Ana Isabel Feliciano remembers crying as she watched doctors wheel her son, identified as A. A. in the suit, off to surgery at Nicklaus to remove the abscesses in his arm and thigh. She and her husband, Armando Alvarez, were terrified.
“You’re putting your 4-year-old through general anesthesia,” Armando Alvarez said, “[when] he should have been enjoying that Christmas break. And instead, we’re … preparing for surgery.”
It was in the waiting room at Nicklaus where Feliciano met another mother whose son was also a patient of Marin-Casariego. They were there for the same reason. That’s when Feliciano and her husband realized it was likely the needle, not the vaccine, that had caused their son’s infection.

Both the Cuervo and Alvarez families are still making sure that their children get the mandatory childhood vaccinations needed for school, but they’re wary.
The new pediatrician for the Cuervo’s daughter, for example, knows not to inject her with McKesson needles. Her mom takes photos of the needles used on her daughter. All three families whom the Herald spoke with believe vaccinations help keep people safe, but they feel like the medical system failed them.
South Florida doctor
Marin-Casariego, the pediatrician embroiled in the lawsuit, has practiced medicine for more than 30 years. She’s listed as having worked as an assistant clinical professor at the University of Miami Miller School of Medicine, with staff privileges at Nicklaus Children’s, Baptist and South Miami hospitals, according to state health department records.
Her attorney, Cook, in an emailed statement to the Miami Herald, reiterated the doctor’s dedication to her patients, casting blame on the pharmaceutical giant.
As soon as Marin-Casariego “first noticed a reaction, she began to investigate and determined that the only common factor with all of the patients who developed reactions was the needles she used,” Cook’s statement reads. “Dr. Casariego denies all responsibility with respect to these infections and reported them to both McKesson and the Florida Department of Health.”
Judge allows suit to continue
In court papers, McKesson’s attorney, Silverglate, tried to have the families’ suit dismissed for failing to state “a cause of action” against the Richmond, Va.-based company. A judge rejected that claim, setting the stage for a jury trial next year in Miami-Dade Circuit Court. In early October, another judge denied McKesson’s motion to dismiss the physician’s cross claim for damages including attorney’s fees.
In court papers, McKesson asserts the families of the infected children may not be able to recover damages from the pharmaceutical firm because the needles complied with what was “generally recognized state of the art at the time.”
McKesson also claims that other entities “may be responsible for the damages alleged in this case,” including initially blaming the Chinese needle manufacturers, the children’s physician, and the producers of the vaccines, alcohol swabs and rubber gloves used in administering them.
McKesson also asserts that the children’s “damages, if any, were the direct result of pre-existing medical conditions ... or as a result of certain circumstances over which [the company] had and continues to have no control.”
The children’s parents say the traumatic experience sticks with them and their kids to this day — the bacterial infections may be gone but the scars are permanent.
“All that fear led to anger when there’s no accountability,” said Sophia Linale’s father, Ricardo. “Big Pharma has some explaining to do.”

