Mpox vaccine worked well, safely, CDC data shows. Committee votes to continue its use.
The mpox vaccine worked well at preventing symptoms and shortening the course of the disease formerly known as monkeypox, and it should continue to be used, a federal panel decided Wednesday.
A global outbreak, which arrived in the United States in May of last year, sickened more than 30,000 and killed 32 in the U.S. before mostly petering out.
Since early this year, cases in the U.S. have trickled in at no more than a handful a week, though officials at the Centers for Disease Control and Prevention were quick to point out that the outbreak continues.
The vaccine, particularly when given in two doses, performed well and appeared safe. CDC experts told the committee in several hours of testimony.
The 14-member Advisory Committee on Immunization Practices then voted unanimously to support continued use of the vaccine in adults during an mpox outbreak. The vaccine can be used on adolescents 12 and up, and data on that age group will be considered in June.

What is mpox?
The mpox virus is a milder version of its relation, smallpox. In most people who were infected, the virus caused blistering, which healed over two to four weeks. Some had more serious disease when the poxes were located in sensitive areas, such as the eye.
The most severely affected were those with uncontrolled HIV who also developed mpox, according to a study published Tuesday in The Lancet.
Until last year, mpox had largely been contained to the African continent. It is not known precisely why the virus jumped first to Europe and then the rest of the world in late spring, infecting 86,000 people in 110 countries, killing 92.
Where did mpox go?: Here's what brought down cases of disease formerly known as monkeypox.
HEALTH NEWS: As COVID turns 3, experts worry where the next pandemic will come from – and if we'll be ready
Only about 4% of cases worldwide have occurred in women; most occurred in men who have sex with men. Those infected have an average age of 34.
According to the World Health Organizations, 43 countries and territories have not detected any new cases in the past three months. Eighteen countries and territories continue to report recent local human-to-human transmission, most of them in the Americas.
Mpox vaccine safety
More than 1 million doses of the Jynneos vaccine were distributed in the U.S., most of them in the late summer. The Jynneos vaccine is intended to be given as two doses one month apart, although some protection was seen in people who received only one.
The vaccine appeared to be very safe, with no problems that hadn't been expected based on a clinical trial, CDC officials said.
Short-term side effects from the shots included redness, pain and swelling at the injection site, pain, fatigue, headache and dizziness. There were very few serious events connected to the Jynneos vaccine.
A small number of vaccinated people suffered myocarditis, a swelling of the heart muscle, after vaccination, but it wasn't clear whether that occurred more often than in the general public.
No pregnant people received the vaccine and too little was known about the immune status of people who received the vaccine to determine its safety in pregnancy or among those with immunocompromising conditions or medications.
Most of the reports about problems with the vaccine were related to the method of giving the shot. Originally, the vaccine was intended to be delivered subcutaneously, into a deep layer tissue in the upper arm, but to conserve vaccine when it was in short supply, that was shifted to an intradermal delivery, just under the outer layer of skin and typically delivered lower on the arm.
Providers are less used to intradermal shots and often delivered the vaccine into deeper layers of skin. These injections also leave longer-lasting scars than the subcutaneous shots, so many people didn't want to receive them.
Because the COVID-19 vaccine has been seen to increase the risk of myocarditis, the two vaccines are not recommended to be given together. Otherwise, the Jynneos vaccine can be delivered with other shots.
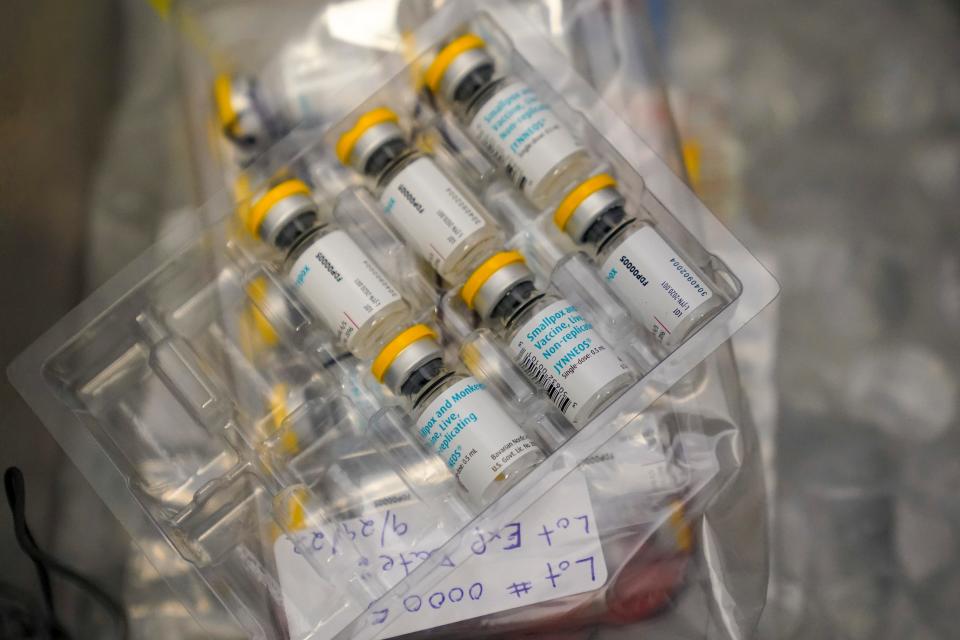
Mpox vaccine effectiveness
Vaccine effectiveness varied by study, but one U.S. study found that people who were unvaccinated were seven times more likely to develop mpox than those who received one dose of Jynneos and nearly 10-times more likely than those who received two doses. There was no difference in effectiveness seen between those who received the shot intradermally versus subcutaneously.
Because behaviors changed during the outbreak and cases also fell in countries where the vaccine was not available, it's not possible to determine the exact benefits of the shots, CDC officials said.
It's also unclear how long protection lasts, the CDC experts said. Studies have suggested the vaccine remains protective for at least two years. One study now underway in the Democratic Republic of the Congo is exploring a booster dose given five years after initial vaccination.
Contact Karen Weintraub at kweintraub@usatoday.com.
Health and patient safety coverage at USA TODAY is made possible in part by a grant from the Masimo Foundation for Ethics, Innovation and Competition in Healthcare. The Masimo Foundation does not provide editorial input.
This article originally appeared on USA TODAY: Mpox vaccine safety, effectiveness: CDC says it worked well, safely

