What newer immunizations mean for this year’s respiratory viral season
- Oops!Something went wrong.Please try again later.
- Oops!Something went wrong.Please try again later.
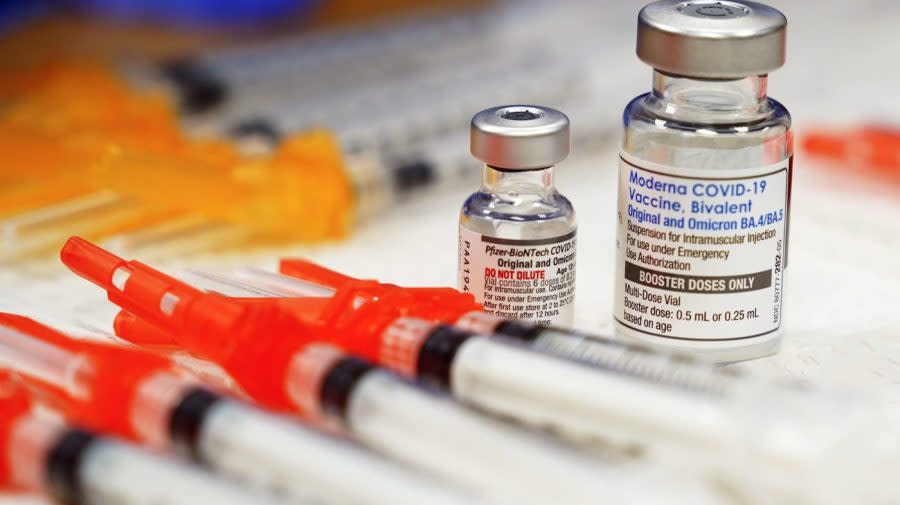
As the fall and winter months approach, a new round of viral protection is expected to become available to the U.S. public: updated COVID-19 vaccines and new preventatives for the respiratory syncytial virus (RSV).
With this arsenal of medicines, the country may be better prepared to take on the respiratory viral season — when diseases like the flu, COVID and RSV typically surge. But the rollout of these new treatments will test how effectively vaccinations can be used in the post-pandemic era.
No updated COVID-19 vaccines have been authorized by the Food and Drug Administration (FDA), though stakeholders are anticipating the shots to become available by the end of this month and for the rollouts to begin as soon as mid-September.
“This season has the same potential that we saw in last season … with having RSV and flu and COVID all at the same time,” Michelle Fiscus, chief medical officer of the Association of Immunization Managers, said. “We’re obviously seeing increasing rates of COVID-19 infections in many parts of the U.S. now so we’re excited to have an updated COVID vaccination.”
Vaccine uptake for SARS-CoV-2 has continually petered out since the first round of immunizations were made available in late 2020, with older age groups consistently having higher vaccination rates. Fiscus expects this next vaccine campaign to be no different, though she said she hopes more people who skipped getting a bivalent booster will come around to this latest shot.
Older adults in the U.S. will also benefit from having access to the first-ever RSV vaccine this year. The FDA approved the shot for use in adults over the age of 60 in May. For infants and toddlers, a preventative monoclonal antibody was approved by the FDA in July.
As a pediatrician, Fiscus said she is excited for the new RSV preventive medicine, but noted logistical and financial issues are already present. The RSV antibody sold as Beyfortus from Sanofi has a list price of about $500 for private payers which can be a financial barrier for doctors offices already stocking up on flu and COVID vaccines.
Stakeholders have a relatively small time frame to administer the RSV antibodies as indicated. The treatment is recommended for infants born during or entering their first RSV season — which typically lasts from mid-September to May — as well as children up to 24 months old who are still vulnerable ahead of their second RSV season.
“Getting the messaging out is going to be a challenge and a really tight turnaround, I think, for the RSV monoclonal antibody,” Anne Zink, president of the The Association of State and Territorial Health Officials (ASTHO) and chief medical officer for the Alaska Department of Health, told The Hill.
“The strategies and techniques the states are taking [are] really different than say the COVID treatment or vaccine,” she added.
Zink says she is working with pediatricians, who already have close relationships with parents, making sure they have the relevant data and information to give their patients.
While Beyfortus is not a vaccine, the immunization committee for the Centers for Disease Control and Prevention (CDC) voted to include it in the federal Vaccines for Children program, which provides free shots to about half the children in the U.S.
According to a Sanofi spokesperson, supplies of Beyfortus will be available beginning in mid-September with doses continuing to be made available throughout the season. When asked about the level of uptake the company is anticipating, the spokesperson said they are working to “help provide millions of parents” with the ability to protect their infants from RSV.
Concerns over timing and logistics are also at the forefront of the COVID-19 vaccine campaign. This will be the first round of COVID immunizations to not be subsidized by the federal government. Efforts by the federal government, however, are underway to let people who want the COVID vaccine to get it.
To ensure continued access to vaccines for the uninsured, the Biden administration announced earlier this year the launch of the Bridge Access Program, a public-private partnership to distribute coronavirus vaccines and treatments through health centers and pharmacies.
Negotiations with pharmacies are ongoing. Multiple outlets recently reported that distribution through pharmacies may be delayed to mid-October.
CDC spokesperson Kathleen Conley told The Hill that pharmacies were always intended to follow after health centers in the Bridge program and an October launch represented an “accelerated timeline under federal contracting rules.”
Industry stakeholders aren’t sure how popular their latest COVID shots will be this season, however. In an earnings call earlier this month, Pfizer CEO Albert Bourla noted vaccination rates in the first six months of 2023 failed to reach the company’s projections, though these injections were for the older bivalent dose.
Citing past vaccination uptake, Bourla anticipated the fall and winter to see many more consumers getting the shot, but “severity of disease and people’s desire for treatment” present continued uncertainty.
He said he expects the updated shot to be authorized some time this month and Pfizer has stated it can begin distribution by the end of August pending authorization.
Messaging surrounding the vaccines will play a key part in convincing people to get immunized. Early on in the coronavirus vaccination campaign, mixed messaging resulted in confusions — such as when President BIden said “You’re not going to get COVID if you have these vaccinations.”.
The CDC later had to work around the absolute nature of his remark, reiterating that breakthrough infections do occur as with all vaccines. The true benefit of the COVID-19 vaccine is its ability to prevent severe illness, hospitalization and death, which experts see as the key point to drive home.
“I think the important information here is No. 1: the vaccine doses clearly will not prevent you from getting infected or even potentially transmitting the virus,” Michael Osterholm, epidemiologist and director of the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota, said. “But it has a major impact on whether you’re likely to become seriously ill, be hospitalized and die.”
“The second thing though is the data becoming increasingly clear that previous vaccination status greatly reduces your likelihood of getting long COVID,” Osterholm said.
Osterholm added that he believed waiting until mid-September to launch a COVID-19 vaccine campaign was a mistake, saying it has not yet been proven that SARS-CoV-2 is a seasonal virus. He called for a faster rollout of both the updated shots as well as the RSV antibody and disagreed with lumping the two viruses together with influenza, due to possible differences in timing.
WIth the newer treatments, informed by past surges and outbreaks, it would be easy to believe that this year’s respiratory viral season will be easier. Still, Zink explained that there are factors at play that can make this year just as harsh as last year.
While quarantine during the pandemic was partially blamed for reducing RSV exposure among children, leading to higher rates of illness and hospitalization, ZInk noted that harsh RSV seasons occurred even before the outbreak. And now the U.S. is entering a season with a drastically shrunken workforce.
“One out of five health care workers have left the field in the last five years. 46 percent of public health workers have left the field in the last five years,” Zink said. “And on top of that, we’re entering a respiratory season that even if it’s a good season with good vaccine uptake and good vaccine matching will probably result in more hospitalizations.”
For the latest news, weather, sports, and streaming video, head to The Hill.

