British patients fly to New York to obtain Alzheimer’s ‘wonder drug’
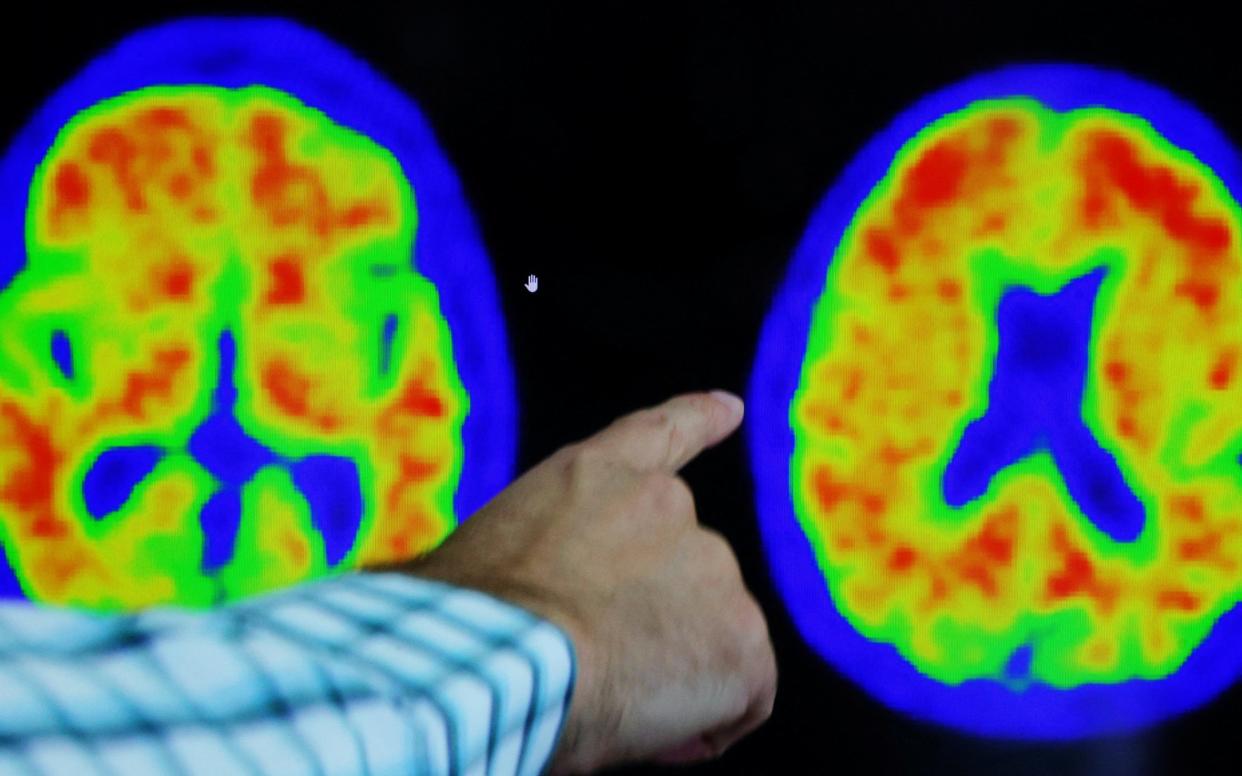
British patients with early-stage Alzheimer’s disease are flying to New York to be treated with new wonder drugs following delays in the UK, an expert has warned.
The breakthrough drugs, called lecanemab and donanemab, have been proven to slow down the progression of the disease by as much as 60 per cent in clinical trials.
They work by helping to remove the buildup of the protein amyloid in the brain and could herald a new era of dementia treatment by tackling its cause rather than just alleviating symptoms.
But while the drugs have been licensed for use in the United States, they are yet to get the green light in the UK and experts say hopeful patients are paying to travel abroad to bypass the red tape.
Sir Prof John Hardy, of University College London (UCL), who was the first to identify the role of misfolded amyloid in Alzheimer’s 30 years ago, told the Guardian: “We now have finally got something that started here in the UK, it would be nice to see it actually helping patients. There is frustration, yes.”
Prof Hardy, who is also vice-president of Alzheimer’s Research UK, said: “I’ve just had two patients phone me up who are flying to New York privately to get the treatment. That’s not a good outcome.”
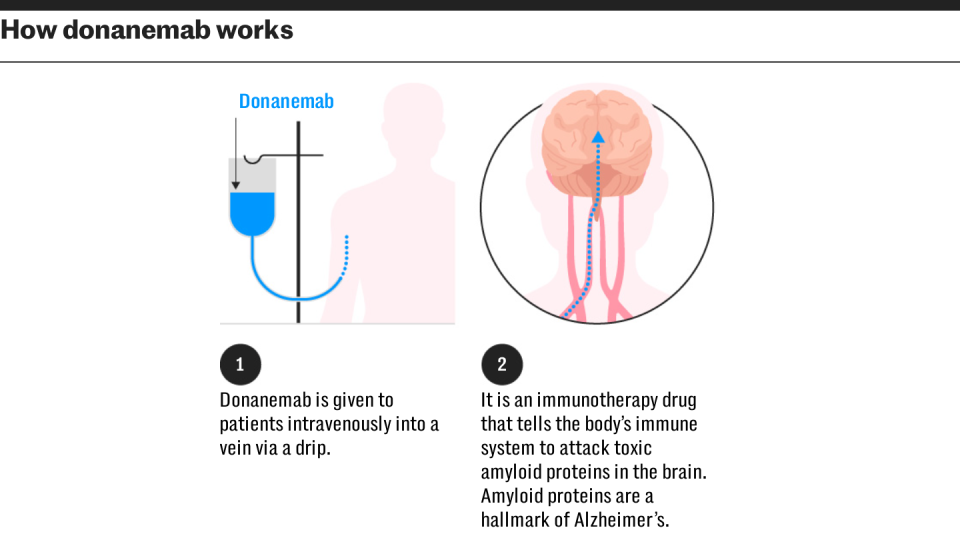
The Medicines and Healthcare products Regulatory Agency (MHRA) is considering applications for lecanemab, developed by Eisai and Biogen, and Eli Lilly’s donanemab.
Concerns have previously been raised about the capacity of the NHS to roll out these drugs at speed given the number that could benefit could be as high as 280,000.
Patients will require MRI scans so doctors can decide if they are eligible to get the drugs.
NHS England has set up a dedicated team to ensure the health service can roll out the drugs quickly.
It said the cost of implementing the new drugs, if and when approved, would be between £500 million and £1 billion per year.

NHS England said patients would need “a baseline MRI scan and then either a PET-CT scan or lumbar puncture that confirms the presence of beta-amyloid proteins in the brain”.
It added that new biomarker-based blood tests may be available in the future to screen patients following trials showing the less invasive tests had promise.
“This means we should be cautious about driving a massive expansion, for example in amyloid PET-CT capacity, when this could become redundant in the longer-term.”

