Is it worth £7000 to be infected with a disease and go into lockdown?

Esmée’s room is newly refurbished, with two floor-to-ceiling windows and panoramic views of the capital’s parks and waterways. There’s a 43-inch flat-screen TV, a PlayStation 5, and cuisine rustled up by a chef who used to work in luxury hotels. When she presses her face to the glass, she can see walkways and quaysides 25 floors below, and when the weekend comes and the kayaks and sailing boats arrive, faces are just about visible.
The building has an on-site gym, coffee shop and waterfront Mediterranean seafood restaurant – although Esmée hasn’t visited any of them because she’s confined to the 225 square metres of her room. Despite being in peak health, her blood and temperature are checked regularly throughout the day – and night; her diet is calorie-controlled; and on one occasion she had to have a pathogen sprayed up her nose. Esmée is one of the latest human guinea pigs who have made it through the competitive screening process at the newest and largest ‘human challenge’ quarantine facility in the world. A human challenge trial is where healthy volunteers are deliberately infected with a virus or bacteria, and can test whether new treatments and vaccines are safe and effective.
Esmée is testing a flu vaccine and will be £3,000 better off by the end of 10 full days in isolation. ‘They don’t lock you in, for obvious reasons,’ Esmée will tell me later – the obvious reason being the law. ‘But only the nurses are supposed to open the door. It’s a mind game: you just can’t leave.’
Run by medical research organisation hVIVO, the facility occupies two floors of Forty Bank Street in the Canary Wharf area, and in terms of its scale is the first of its kind. Dubbed ‘FluCamp’, it’s part hotel, part human laboratory. Many of the 50 bedrooms and en-suite bathrooms have spectacular views of London’s skyline and the Thames, but ‘guests’ sleep on hospital beds and room service is delivered by nurses in the sort of space-age face shields that conjure up Covid wards during the pandemic. As well as the kitchen, which produces restaurant-quality three-course meals, there is also a virology laboratory where technicians process and analyse hundreds of samples of blood and nasal swabs.
Historically, these studies have taken place in academic institutions and teaching hospitals, but in recent years the pharmaceutical industry and small biotech companies, keen to develop new vaccines and antivirals in the wake of the Covid-19 pandemic, have been turning to human challenge trials. They are cheaper and faster than the alternative, field trials, where volunteers receive the vaccine and return to their daily lives in the hope they are exposed to whatever virus they’ve been vaccinated against. More than 44,000 people took part in the field trial for Pfizer’s Covid-19 vaccine. A challenge study, on the other hand, requires 40 to 200 people and can be completed in close to a year.
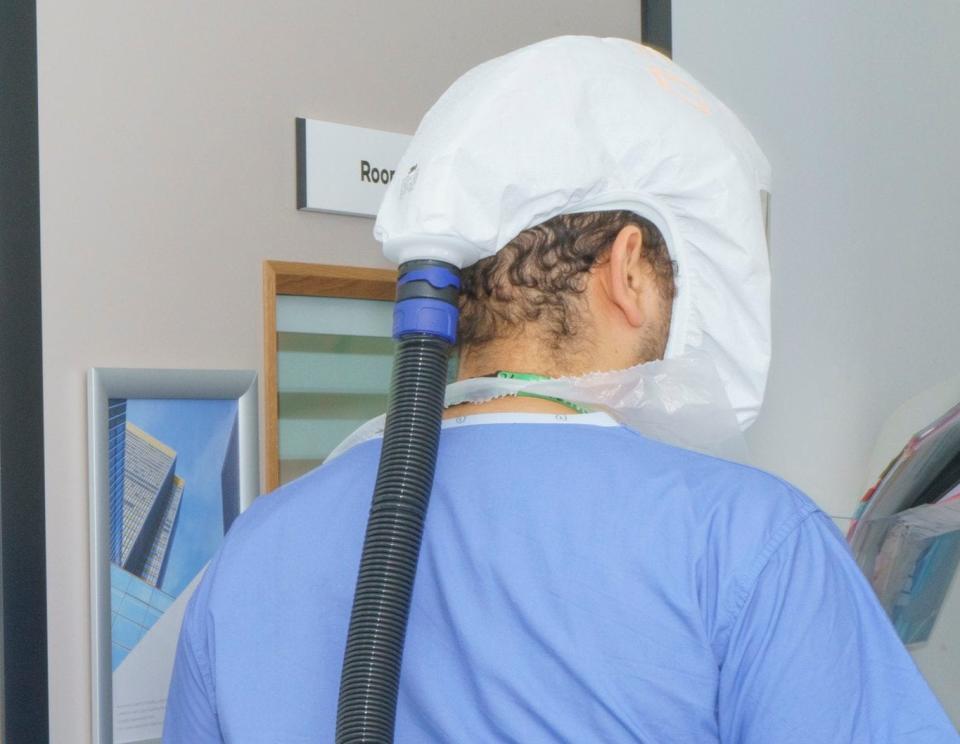
In non-pandemic times, a field trial generally takes two to three years. Human challenge trials, therefore, can shave years off the time it takes to bring a vaccine or treatment to market, which is where hVIVO (formerly Open Orphan) comes in. It’s currently the only company in the world to specialise in these kinds of trials and played a key part in the world’s first human challenge trial for Covid-19.
In 2021, 36 young people were inoculated with SARS-Cov-2 to learn more about the disease, in a study commissioned by the UK Government. It was a time of heightened risk. More than 73,000 people had died from Covid-19 in England and Wales in 2020. ‘Fortunately, we didn’t need to test a vaccine against the disease, as by the time the trial was completed, it was clear that the Pfizer, Moderna and AstraZeneca vaccines were being successful and coming into licence,’ says Andrew Catchpole, hVIVO’s chief scientific officer.
Before the swanky Canary Wharf facility opened in April, hVIVO had a small centre in London’s East End, and a facility with more beds across the road, but it started by running trials in hotels. Unbeknownst to guests, hVIVO would take over an entire floor of a ‘well-known, large hotel chain. Within 72 hours we’d have converted it into a challenge study suite,’ says Catchpole, who says there was ‘no risk’ to hotel guests.
Its big success to date: the vaccine against respiratory syncytial virus (RSV), which poses a huge threat to the very young and very old. The Pfizer vaccine was approved last year. ‘We’re a commercial organisation but we think we do good,’ says Yamin Khan, hVIVO CEO.

But it also makes a lot of money: revenue of £56 million in 2023, a 15.5 per cent increase on £48.5 million in 2022. In three years, from 2021 to 2023, the value of its contracts almost doubled, from £46 million to £80 million. Big pharma accounts for 40 per cent of business – Merck, Pfizer, GSK; 60 per cent is smaller biotech companies. The target is an annual revenue of £100 million by 2028. The new facilities in Canary Wharf, which reportedly cost several million pounds to kit out, will allow hVIVO to run more trials. But more trials need more volunteers.
These volunteers are screened at the company’s recruitment centre in Whitechapel, a diverse area of east London. (It has a second recruitment centre in Manchester.) The waiting area has the feel of a GP’s surgery – crowded, bursting with people. Three administrative staff members working behind a counter are passing out documents and clipboards. Notices on the wall urge people to ‘Please drink 3 cups of water before your appointment today’. And, in case anyone was in any doubt about what was about to happen, staff wander in dressed in green scrubs. Visit one is a blood test. If you pass, you’re invited back for visit two, a full medical. If that goes well, you’re judged a healthy volunteer ready to take part. ‘Compensation’ – volunteers are paid but hVIVO never talks of ‘wages’ – is up to £4,400 for a 16-day trial. The cost-of-living crisis has changed the demographic, according to Melanie Smyth, director of clinical operations. ‘I’ve been here just over a year, and when I joined it was predominantly young professionals and university students. Now, there’s a lot more older people – mid-30s to 50s.’
Most, however, won’t be accepted for the trial. There are so many grounds on which to be eliminated: high blood pressure; low blood pressure; allergies; mild asthma; gallstones; anyone on diazepam, or ‘maintenance medication’, for example statins, or taking recreational drugs. A GP letter is required with a full medical history – ‘so they can’t lie’, says Smyth. ‘We need to know if they’ve had their spleen removed.’ What’s more, people react differently to being stuck in a room on their own for weeks. Anyone with a history of claustrophobia, anxiety or psychosis is also ruled out.

But the biggest barrier is antibodies. Most of hVIVO’s work is with respiratory viruses, such as influenza, HRV (human rhinovirus) and HMPV (human metapneumovirus). The purpose of its trials is to test if an antiviral drug or vaccine works. ‘We’re trying to address unmet medical needs,’ says Catchpole. In other words, the pharmaceutical industry’s hunt for a blockbuster drug with huge market potential. Take flu vaccines. ‘Currently, the flu seasonal vaccines are not very efficacious and even the companies wouldn’t pretend they are,’ says Catchpole. A universal flu vaccine that protected against any strain would be the holy grail for big pharma.
But colds and flu, as we know, are common. And the ubiquity of these viruses presents a problem. ‘The majority of volunteers who come through our door are excluded because they already have too high levels of antibodies and would not get sick if we gave them the virus,’ says Catchpole. It turns out that flu antibodies can last a lifetime – 90 years plus, according to a paper in science journal Nature in 2008. Last year, hVIVO screened 17,000 healthy volunteers; fewer than 1,000 made it on to a trial.
So when Esmée walks into the foyer of Forty Bank Street with a rucksack and yoga mat on 15 May, she feels she’s one of the lucky ones. When I meet her before she enters the trial, she’s excited. A social media manager for a charity and a sailing enthusiast, she’s been living on a boat moored in Portishead Marina in Bristol with her boyfriend since December 2022. They plan to sail across to the Caribbean in September, part funded by her stint in quarantine. Esmée first found out about FluCamp via a TikTok video and was ‘intrigued’. She attended two screenings and two months ago received an email confirming her place on a trial to test a flu vaccine. ‘I was so pleased I was eligible,’ she tells me. ‘I won’t get cabin fever if I can have a nice view of London.’

A month before entering the facility, Esmée was given the vaccine being trialled. Her arm was covered so she couldn’t see if it was the placebo or the real drug. On the third day in isolation she’ll be infected with flu. The details of the trial are a closely guarded secret. She won’t be allowed to tell me if she gets flu or even discuss any symptoms.
For the three days leading up to entry, she’s been focused on removing toxins from her body: no alcohol, no coffee, as both can ruin the study. She’s also had to limit ‘intense activity’ – an elevated heart rate can also impact results. ‘I’ve been struggling,’ she admits, given her gym and coffee habit. But she’s made plans for how to pass the time: gentle yoga, reading, online study for her day-skipper qualification, and work. ‘Hopefully the routine will keep me sane.’
Esmée ascends to the 25th floor to enter FluCamp. The doors open and light floods into the sleek interior. The ‘crash trolleys’ for emergency resuscitation and the used tissues (volunteers blow their noses and unfortunate staff members weigh the discharge) are tucked away. The bedrooms, the size of Manhattan hotel rooms, run along each side of long corridors.
But not all the rooms have a window – 14 face inward with no natural light. Occupants have a mural of an alpine or beach scene to look at instead.

There have been complaints, admits Adam French, vice president of clinical operations. He explains that rooms are allocated randomly on admission. ‘Each study needs to be grouped together,’ he says. Some will get inside rooms; some outside. Some people actually choose not to have a window, he says – acrophobics (a fear of heights) or gamers who stay up all night and sleep all day. (Staff admit people can get grumpy when they’re woken for a test.)
The nurses’ desk is the nerve centre of the operation. A large screen lists the new arrivals, including Esmée, along with any confiscated items – such as chocolate. ‘Yes, we check their bags,’ says French, ‘to make sure they haven’t got anything that’s restricted in the study.’ Participants know chocolate is banned but still sneak it in, he says. The cocoa in chocolate is a stimulant. Vitamin pills are forbidden, as are steroid face creams. Eight of the new arrivals have already been sent home after the screening for pathogens, nicotine and recreational drugs. There are now 14 males and seven females left – ranging from 21 to 43.
In the kitchen, John Thompson, who previously worked in luxury hotels including the Landmark London, shows me the menu: leek and potato soup, sweet and sour chicken. Areas are colour-coded: amber for the corridors and red for bedrooms, which are off-limits unless you’re wearing gold-standard PPE or are a ‘guest’. Negative air pressure ensures pathogens are contained in the bedrooms, even when you open the door. The consequence of a malfunction is easy to grasp: the virus would escape.
I leave Esmée in room 23 but keep up with her journey via TikTok videos of her experience. Volunteers are allowed phones but all posts are monitored to make sure nothing confidential is disclosed. I hear about the French onion soup and falafel and potato salad – ‘Both really tasty!’ On day three she describes being given the flu. ‘I had to wear goggles, while the doctors dropped some liquid up my nose, and a clip for another half-hour to make sure I was actually being infected.’

Are there risks to Esmée’s forced infection? In 2006, six healthy young men who took part in a trial at Northwick Park Hospital, London, for an experimental treatment for diseases including rheumatoid arthritis and multiple sclerosis, had to be rushed to intensive care after suffering life-threatening reactions. They were hospitalised for weeks and one subject had parts of his fingers and feet amputated. The ‘Elephant Man’ trial, as it became known, was a Phase 1 trial, points out Catchpole, the first time a drug is put in a human. hVIVO does the second phase of trials to check the best dose of the treatment and find out more about side effects. (The company now also offers Phase 3 clinical field trials, where volunteers are given a new treatment – and sometimes a placebo – and sent home. Phase 3 trials involve hundreds, sometimes thousands, of volunteers and aim to assess how well the new treatment works.) ‘There’s inherent risk in doing any clinical research,’ Khan admits, ‘but doing Phase 2 is less risky than Phase 1.’
Still, people on a challenge trial are not only being exposed to an experimental treatment that might previously have only been tested on 40 or so people, but also to the disease itself. Usually, deliberate exposure to harm is considered a contravention of the Hippocratic oath, but the ethics blur when it comes to research for cures. Volunteers are told the worst that can happen and sign a waiver before the trial.
One former volunteer admits the idea of being a human guinea pig was hard on the nerves. Cezary, 25, a film and television graduate, who took part in a trial in January at hVIVO’s former, less appealing site, remembers ‘infection day’ as a moment of high drama. ‘I had to lie on my back with my head on the edge of the bed, tilted back. I was surrounded by four nurses in space-age helmets and masks. One put droplets of pure virus into my nostrils. It seemed very surreal.’
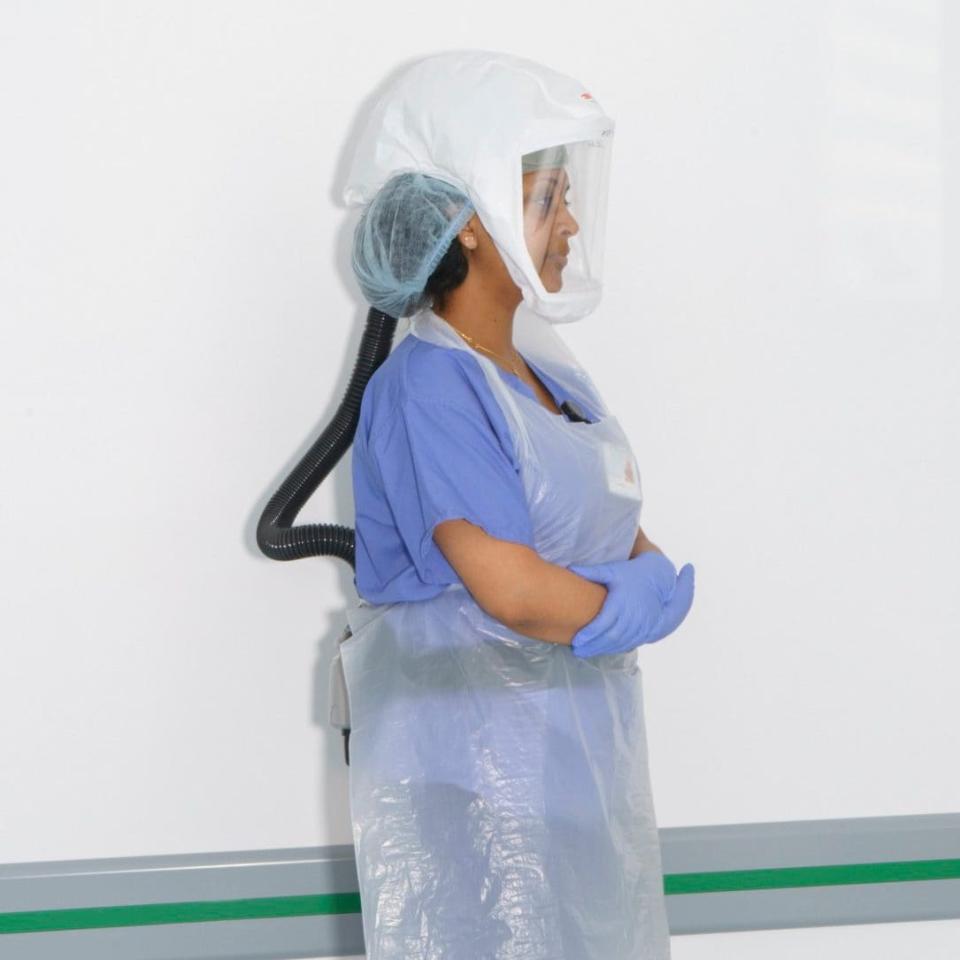
Cezary, who lives in London, had found it hard to survive in the film industry without family backing. He’d had to scale down his hopes and work in a bar. He got paid £2,950 for the 10-day trial – a sum that would have taken over two months to earn in the bar. ‘The money was so good I thought there must be some kind of catch. I thought that maybe I’d leave the trial with a third lung growing out of my body. Maybe I’d have lots of different medicines tried on me, or I’d get sick afterwards… But it turned out there wasn’t a catch and I’m really happy with that because I was struggling financially.’
He had two ‘intense’ days when he was cannulated for the whole day so researchers could take lots of blood. ‘If you’re not good with needles and giving blood, you shouldn’t do this,’ he advises. The daily nasal swab was also unpleasant. ‘The nurses go as deep as they can until you tear up.’ He did yoga, painted, read, crocheted a beanie, did some voluntary work and became obsessed with the reality TV series Glow Up: Britain’s Next Make-Up Star. And although he felt hemmed in (‘my room was facing the back of the building’) and found the isolation hard (‘I felt like I was stuck in this perpetual state of hospital’), he got what he needed out of it. ‘It’s about money at the end of the day, and I say that without shame. I’m going to do it again.’ hVivo recommends six months between studies. Last week, Cezary received an email saying he was eligible for another trial.
FluCamp at Forty Bank Street, however, is certainly an upgrade. Mason, 31, an unemployed media marketing officer, says he felt like he’d been on holiday. ‘I got a fantastic view. I’d watch the sun rise in the morning and set in the evening.’ He read motivational books and says having a private room with a television and PlayStation and no housemates was ‘bliss’. ‘It’s a very easy way of getting money and, believe it or not, I’m sick of colds and flu – and if that can help, why not do it?’ he says.
After 10 days in quarantine, it’s time for Esmée to leave. She’s understandably chipper to be out. ‘For the first few days everything was new, exciting, interesting,’ she says. ‘By day five I knew the routine. By day nine I was ready to come home. No one can prepare you for the isolation. I wish I’d brought a few more things to do.’ She longed for something that wasn’t a screen, like sudoku, and says life became very food-orientated. ‘If you have a meal you don’t like, it’s sad,’ she explains, citing last night’s mushroom pasta. But mostly, what kept her going was the money. ‘Obviously it’s incredibly important to help science, but I can’t lie, I wouldn’t have signed up otherwise. I was thinking about the Caribbean.’

