Novavax (NVAX) Down on COVID Jab-Manufacturing Problem Reports
Shares of Novavax, Inc. NVAX were down almost 15% on Wednesday following a Politico report, which claimed that the company was facing core manufacturing problems related to its COVID-19 vaccine candidate. Per the report, Novavax’s method to test the vaccine’s purity has not met regulators’ standards, and could take a lot of time before it is set right.
However, Novavax issued a statement to reaffirm its commitment to deliver a high-quality vaccine that meets the strict standards of production and manufacturing. The company believes that its COVID-19 vaccine will be ready by the end of this year.
Novavax plans to complete rolling regulatory submissions for its COVID-19 vaccine candidate in the United Kingdom, Europe, Canada, Australia and New Zealand in the next few weeks. The company has already commenced its regulatory filings seeking emergency use of its vaccine in India, Indonesia and the Philippines.
The company has also filed for Emergency Use listing with the World Health Organization last month, and plans to submit an Emergency Use Authorization (“EUA”) request with the FDA by 2021-end.
Shares of Novavax have rallied 22.7% so far this year against the industry’s decline of 10.9%.
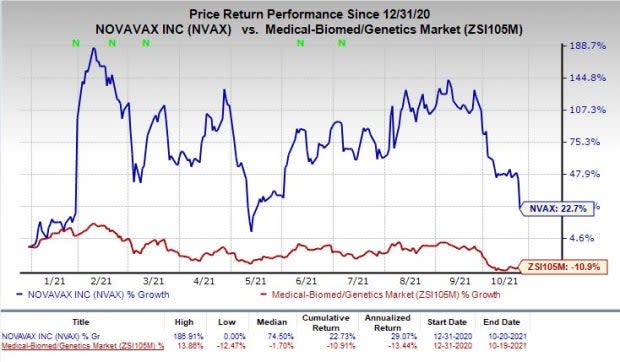
Image Source: Zacks Investment Research
Based on data from a late-stage study announced in June 2021, the company’s COVID-19 vaccine candidate demonstrated an overall vaccine efficacy of 90.4% and achieved 100% protection against moderate and severe coronavirus disease. The COVID-19 vaccine candidate was found to be effective against strains of the coronavirus first found in the United Kingdom, the United States, Brazil, South Africa and India.
Novavax’ candidate, NVX-CoV2373, is also being evaluated in studies in adolescents aged between 12 years and less than 17 years.
The company aims at manufacturing 150 million doses per month by the end of 2021. It has also signed advance purchase agreements with different countries and organizations for supplying more than one billion doses.
We note that Pfizer PFE / BioNTech’s Comirnaty and Moderna MRNA were granted EUA by the FDA in December last year. J&J JNJ received EUA in the United States for its single-shot COVID-19 vaccine in February this year. Although AstraZeneca’s COVID-19 vaccine is yet to be authorized in the United States, it is available in multiple other countries.
We note that Novavax’s COVID-19 vaccine is lagging the race. Novavax plays a key role for the COVAX Facility as the latter is dependent on the company to vaccinate low-income groups. Novavax has partnered with the Serum Institute of India to jointly supply 1.1 billion doses of COVID-19 vaccine candidate to Gavi through the COVAX facility.
Zacks Rank
Novavax currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Novavax, Inc. (NVAX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
