OPNT: Research and Development Meeting Highlights Development Products…
NASDAQ:OPNT
READ THE FULL OPNT RESEARCH REPORT
Business Update
R&D Meeting Highlights Pipeline of Addiction and Drug Overdose Treatments
On October 17, 2019, Opiant Pharmaceuticals, Inc. (NASDAQ:OPNT) held a research and development meeting to highlight the companies most advanced programs for treating addiction and drug overdose. The slide presentation for the event can be found here.
OPNT004 – Drinabant for Treating Acute Cannabinoid Overdose
Dr. Phil Skolnick began the event with an overview of OPNT004 (drinabant), a CB-1 antagonist, for the treatment of acute cannabinoid overdose (ACO). The following figure shows the number of states that have legalized cannabis in one form over another the past few years. This has resulted in an increase in the number of emergency department visits for cannabinoid overdose from approximately 500,000 in 2011 to more than 1.7 million in 2019. This number is expected to continue rising as the number of states that legalize cannabis continues to grow.

The experience of Colorado serves as a good proxy for what can occur following the legalization of cannabis in regards to increased ED visits. The following graph shows that following legalization of cannabis in Colorado in 2011 there was a substantial increase in the number of hospitalizations related to marijuana in the following years.

Cannabinoid (CB-1) receptors were first identified in 1990 and multiple high affinity compounds were subsequently produced to study the biology of CB-1 receptors. A number of pharmaceutical companies developed CB-1 antagonists for multiple indications (e.g., obesity, addiction, schizophrenia). Rimonabant, a CB-1 antagonist, was briefly marketed in Europe (2006-2008) as an anti-obesity agent, however it was removed from the market in 2008 due to psychiatric side effects following chronic use.
Drinabant, which is structurally distinct from rimonabant, was initially developed at Aventis (later at Sanofi-Aventis following the merger). Following the withdrawal of rimonabant development of drinabant was halted, although it had been tested in >700 subjects in various Phase 1 and 2 clinical trials. An extensive oral toxicology data set also exists for the drug including 4- and 13-week toxicity in rats and dogs, 13-week toxicity in mice, 6-month toxicity in rats, and 1-year toxicity in dogs. Opiant licensed drinabant for the treatment ACO from Sanofi in December 2018. A manufacturing contract was signed with Sanofi in July 2019.
An assortment of data supports the use of a CB-1 antagonist for the treatment of ACO. The following slides show the results of administering THC to rodents, which causes a drop in body temperature when it is given either through injection or inhalation. This effect is mitigated by pre-treating the animals with a CB-1 antagonist. Additional behavioral effects of THC, such as locomotion, catatonia, and analgesia can also be reversed by CB-1 antagonists.

Clinical evidence also exists for the ability of CB-1 antagonists to block the effects of THC. Sanofi conducted a study of drinabant (then called AVE1625) that showed its ability to offset different effects of THC when administered prior to THC dosing. The following figures show that when administered three hours prior to increasing amounts of THC, drinabant offsets the effect of THC on body sway, elevations in heart rate, ‘feeling high’, and ‘altered passing of time’.

In an acute overdose situation, it isn’t possible to administer an oral medication, thus Opiant will be developing OPNT004 as single use injectable therapy. This should simplify and accelerate the development timelines since there will not be any psychiatric issues associated with chronic use of CB-1 antagonists (such as those that caused the withdrawal of rimonabant).
OPNT003 – Intranasal Nalmafene to Treat Opioid Overdose
Dr. Roger Crystal discussed the development of OPNT003 (intranasal nalmefene) for the treatment of opioid overdose. The following chart shows the evolution of the opioid crisis in the U.S., which has gone through four phases in which opioid related deaths were first associated with the increased use of opioid painkillers, then an increase in heroin-related deaths, and now 55% of opioid deaths involve the presence of fentanyl.

Fentanyl is 50x more potent than heroin due to the fact that it is more lipophilic than heroin (which leads to much faster brain penetration) as well as affecting respiration more than analgesia. In addition, fentanyl has a longer duration of action than heroin (>7 hours vs. 1-2 hours). Lastly, fentanyl is very easy to produce since it is synthetic. This increased potency means that while naloxone can be effective in reversing overdose due to fentanyl, multiple and continued doses are sometimes necessary for rescue.
Based on the increased prevalence of fentanyl overdoses, the NIH has called for “…stronger, longer-acting formulation of opioid antagonists.” Nalmefene is an opioid antagonist that has a number of positive attributes that make it better suited to reversing opioid overdose due to fentanyl. It was originally approved by the FDA in 1995 for the treatment of opioid overdose as an injectable (Revex®). It was withdrawn from the market in 2008 for commercial reasons.
Nalmefene is up to 10x more potent than naloxone based on the results of a functional assay using cloned human μ opioid receptors. This increased potency translates to increased efficacy in a carfentanil overdose model in rats. The following figure shows the time it takes to reverse loss of righting reflex (LORR) in rats after administration of carfentanil and nalmefene or naloxone five minutes later. Even at lower doses (9.4 and 18.8 μg/kg), nalmefene reverses the effect of carfentanil overdose similarly to a 150 μg/kg dose of naloxone.

OPNT003 is being developed as a rapid acting, long-lasting opioid overdose treatment. The following chart shows the effect of intranasal nalmefene both with and without INTRAVAIL®, which is a broad class of chemically synthesizable transmucosal absorption enhancement agents to allow the intranasal administration of therapeutics up to 30,000 Daltons molecular weight. The data shows that INTRAVAIL® enhances the absorption of nalmefene when compared to intranasal administration without INTRAVAIL® and that therapeutic doses of nalmefene are present for an extended period of time.

Looking at a close-up of the data that shows only the first hour following administration, it is clear that nalmefene with INTRAVAIL® is rapidly absorbed (T50 of 7 min), which is particularly important given the shortened window of opportunity to treat a fentanyl overdose.

In summary, intranasal nalmefene has a number of characteristics that make it superior to intranasal naloxone as a treatment for fentanyl overdose, including the increased affinity at the μ opioid receptors, a much greater half-life (which can help avoid re-narcotization), and a faster rate of absorption.

Lastly, the potential market opportunity for OPNT003 is substantial. Opiant is focused on four addressable markets: 1) the first responder market (EMTs, police force, fire dept.) is key as they are typically the first person at the scene of an overdose; 2) both patients with opioid use disorder as well as their family members; 3) co-prescribing with opioid painkillers (there are currently nine states with mandatory co-prescribing laws); and 4) civil defense, in which the government is concerned about the potential use of fentanyl as a chemical weapon and thus could stockpile nalmefene for use in the event of an attack.
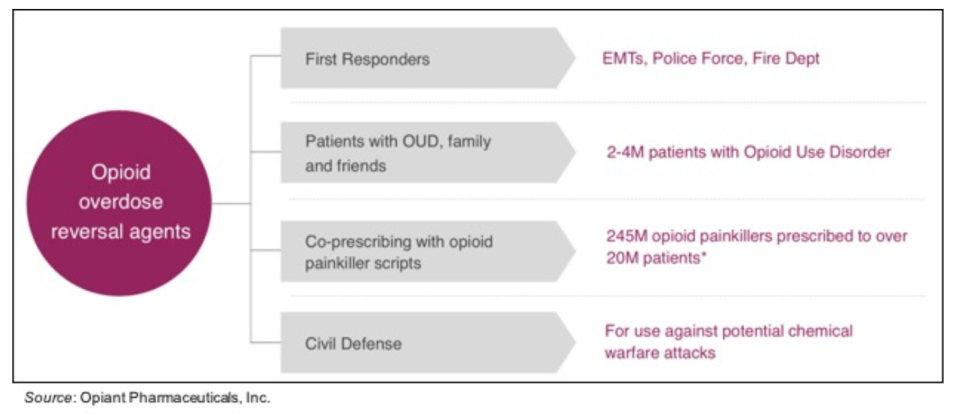
Opiant will be initiating a confirmatory PK study for OPNT003 in the fourth quarter of 2019, with a data readout in the first quarter of 2020. We anticipate an NDA filing in the fourth quarter of 2020 and potential approval of OPNT003 in the middle of 2021. As a reminder, the development of OPNT003 is being supported by a $7.4 million grant from the National Institute on Drug Abuse (NIDA) and a $4.6 million contract with the Biomedical Advanced Research and Development Authority (BARDA), which should support its development through the NDA filing.
OPNT002 – Nasal Naltrexone for the Treatment of Alcohol Use Disorder
Dr. Skolnick discussed the development of OPNT002 (intranasal naltrexone) for the treatment of alcohol use disorder (AUD). Approximately 16-17 million individuals in the U.S. suffer from AUD, but only a very small percentage of them receive any type of pharmacotherapy (<5%). The company believes that a more effective pharmacotherapy would increase the number of patients on medication.
Just as with intranasal nalmefene, Opiant is developing intranasal naltrexone with INTRAVAIL® to rapidly increase the plasma concentration of the drug following dosing. The following table shows that intranasally administered naltrexone with INTRAVAIL® has a Cmax that is approximately 50% higher than orally administered naltrexone along with a Tmax of approximately 12 minutes, and a short half-life. All of these characteristics are suitable for developing OPNT002 for ‘as needed’ intranasal dosing.

The FDA has changed its stance on endpoints for treating AUD. Whereas previously the agency would require an endpoint that examined abstinence (0 drinks), research shows that is an unrealistic expectation given how many different signaling pathways alcohol affects. Thus, the FDA now considers a “harm reduction” endpoint acceptable in AUD trials. For example, a decrease in ‘heavy drinking days’, defined as ≥ 4 drinks in one day for women or ≥5 drinks in one day for a man, as an acceptable endpoint. This is based on a meta analysis showing a couple of drinks a day actually reduces overall mortality and that the decreased risk of mortality effect is lost at approximately 4 drinks per day for women and 5 drinks per day for men, as shown in the following figure.

One of the biggest issues with AUD trials is the high placebo response. In an effort to mitigate this effect, Opiant will be utilizing a Sequential Parallel Comparison Study Design for the Phase 2 trial of OPNT002 in AUD. An overview of the trial design is shown below. During stage 1, two doses of the drug are tested along with placebo. At the midpoint, the trial is unblinded and those that were administered placebo are re-randomized. Subjects that responded are maintained on placebo, while non-responders to placebo are randomized between placebo and active.

In summary, OPNT002 is being developed for dosing “as needed” when a patient anticipates drinking or is craving alcohol. Patients enrolled into the study will not be abstinent drinkers, making it both easier to enroll patients and to meet the primary endpoint of reduction in heavy drinking days. Lastly, the Sequential Parallel Comparison Study Design should help to reduce the placebo response, which is a known issue in AUD studies.
Additions to Management Team
On September 17, 2019, Opiant announced the appointment of Aziz Mottiwala as Chief Commercial Officer and Rahsaan Thompson as General Counsel. Mr. Mottiwala was previously Senior Vice President and Head of Commercial at Avanir Pharmaceuticals as well as Vice President of Marketing Allergan’s Eye Care Franchise. Mr. Thompson was previously Vice President, Law, for Actelion U.S. and previous to Actelion he worked for the law firm Quarles & Brady in Chicago, IL. Both Mr. Mottiwala’s and Mr. Thompsons’ experience will be vital as the company moves toward potential commercialization of OPNT003.
Financial Update
On November 12, 2019, Opiant announced financial results for the third quarter of 2019. The company reported revenues of $20.6 million for the three months ending Sep. 30, 2019, which consisted of $20.5 million in royalty revenue from the sale of NARCAN® Nasal Spray, including a milestone payment due to the company of $13.5 million based on NARCAN sales exceeding $200 million for 2019, and $0.1 million from grant and contract revenue. This compares to revenue of $4.2 million for the three months ending September 30, 2018. The increase was due to increased sales of NARCAN® Nasal Spray by Emergent BioSolutions (EBS) during the third quarter of 2019, which totaled $75 million. Emergent has raised their guidance for full-year 2019 revenues for NARCAN® Nasal Spray to $275-$285 million, and we have increased our estimate to $283 million, which would result in Opiant receiving approximately $7.6 million in royalty income in the fourth quarter of 2019.
G&A expenses for the third quarter of 2019 totaled $3.2 million compared to $2.9 million for the third quarter of 2018. The increase was primarily due to an increase in personnel expenses, royalty expense, and consulting fees partially offset by a decrease in stock-based compensation expense. R&D expenses were $1.8 million for the three months ending Sep. 30, 2019, which was in-line compared to the same period in 2018. Royalty expense for the third quarter of 2019 was $4.9 million compared to $0.5 million for the third quarter of 2018. The increase was due to a $3.1 million increase in payments to Net Profit Partners for the royalties and sales milestones received from the sales of NARCAN® Nasal Spray and a $1.3 million payment made to buyout the net profit interests in nasal nalmefene held by investors who initially supported the development of NARCAN® Nasal Spray.
Opiant exited the third quarter of 2019 with approximately $23.2 million, which does not include the net milestone payment of $10.8 million anticipated from Emergent. The net milestone payment is $10.8 million due to a $2.7 million deduction from the $13.5 million milestone earned to pay for Opiant’s portion of a third-party license agreement entered into between Adapt Pharma and another company. Opiant’s current cash position also does not include the remaining amounts due from the NIDA grant and the BARDA contract for the development of OPNT003. We now estimate that the company will exit 2019 with approximately $30 million.
Conclusion
Opiant is in very strong financial shape with the incoming royalties from the sale of NARCAN® Nasal Spray, and the drug continues to perform ahead of expectations. Now that the company has earned the final milestone payment from Emergent, attention turns to the pivotal PK study for OPNT003 that will likely initiate in the fourth quarter of 2019. With the rate of deaths attributable to fentanyl continuing to rise, there is an urgent need for an easy to administer, potent, and long-lasting opioid overdose reversal agent, and we believe OPNT003 is exactly what is needed to combat the increasing prevalence of fentanyl in the country. We look forward to hearing the results from the PK study of OPNT003 and are glad to hear that the company remains on track to file an NDA in the fourth quarter of 2020. Our valuation remains at $44 and we continue to see the potential for significant upside for investors as the stock continues to trade at a deep discount to that valuation.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks provides and Zacks receives quarterly payments totaling a maximum fee of $30,000 annually for these services. Full Disclaimer HERE.

