Owlet stops selling baby-monitoring sock after FDA warning
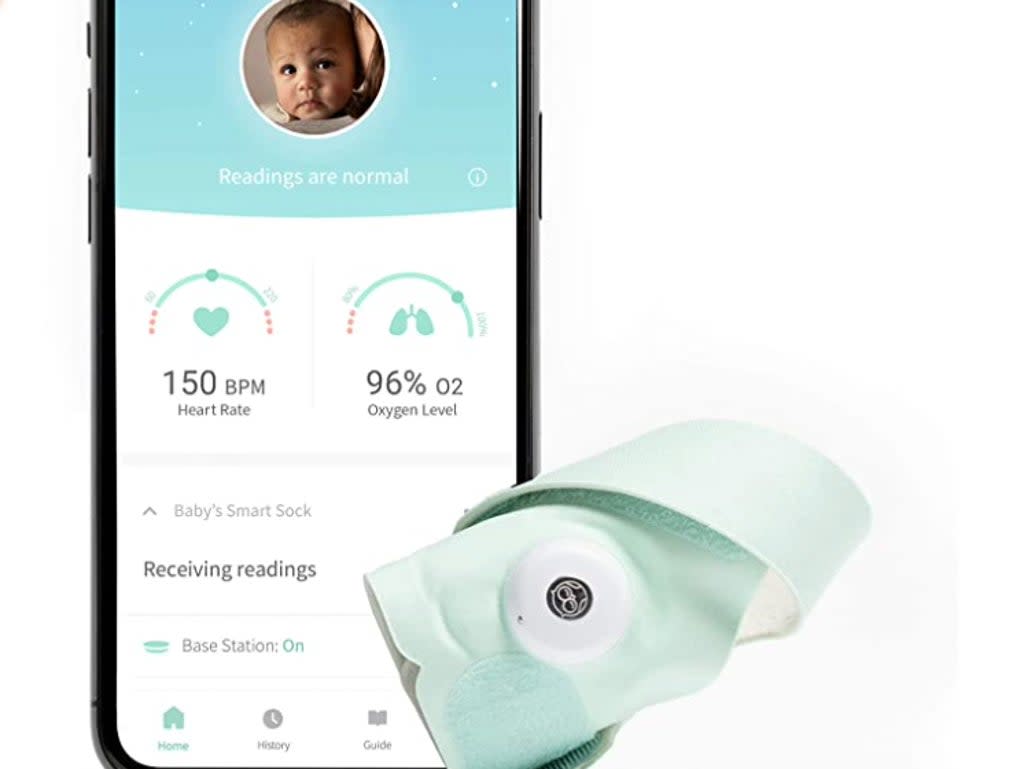
A company that sells baby-monitoring socks has discontinued the product and removed it from its website after a letter from the Food and Drug Administration.
Last month, the FDA issued a warning letter in which it stated that the Smart Sock, sold by Owlet, was actually a medical device, and that the company had been selling the socks without the FDA’s “marketing approval, clearance, or authorisation”.
According to the FDA, the products, which track a baby’s oxygen levels and heart rate, are intended for use “in the diagnosis of disease or other conditions or in the cure, mitigation, treatment, or prevention of disease, or to affect the structure or any function of the body”.
In the letter, the FDA also noted that the baby product company would have to obtain approval or clearance for the device to continue legally marketing it.
This week, Owlet announced in a letter to its own website that it would no longer be selling the Smart Sock products.
In its response, the company acknowledged that the FDA’s letter “did not identify any safety concerns about the Smart Sock,” but rather asserted that the product should be classified as a medical device.
According to the company, based on the FDA’s warning letter, it now plans to pursue “marketing authorisation from the FDA” for the heart rate and oxygen features.
More follows…
Read More
9 best baby monitors for peace of mind, from video to audio models
Video of tree crashing into home and almost hitting five-month-old baby captured by baby monitor

