Oxford Covid-19 vaccine Q&A: How effective is it, and how is it different to the Pfizer vaccine?
Boris Johnson has pledged that the NHS is committed to offering a vaccination to everyone in the top four priority groups by Feb 15, promising that people in England will soon be within 10 miles of their nearest vaccination centre.
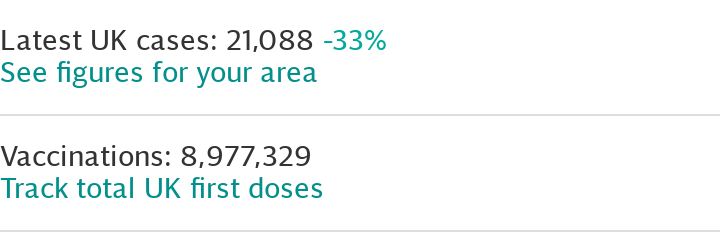
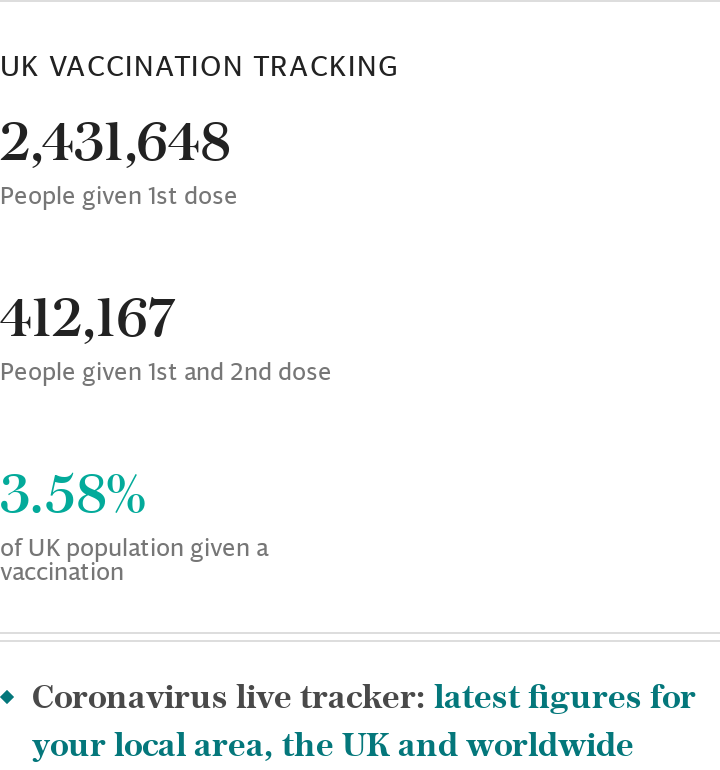
As of Jan 25, more than seven million people in the UK have had at least one Covid vaccine since the programme was launched, official figures show.
Almost four in five aged 80 and up have been vaccinated in England. While vaccinations have now begun in priority groups three and four, which includes those in their 70s - some 5.6 million people - and people listed as clinically extremely vulnerable.
The Pfizer/BioNTech vaccine and Oxford/AstraZeneca vaccine are the two jabs currently in rotation in the UK, with doses developed by Moderna, which was approved on Jan 8, expected to arrive by the Spring.
Brian Pinker, 82, was the first person to be vaccinated with the Oxford/AstraZeneca Covid-19 vaccine at Oxford University Hospitals NHS Foundation Trust's Churchill Hospital on Jan 4.
The vaccine gained regulatory approval in December. "The Government has today accepted the recommendation from MHRA to authorise Oxford University/AstraZeneca's Covid-19 vaccine for use," reads the statement issued by a Department of Health and Social Care spokesman last year.
"This follows rigorous clinical trials and a thorough analysis of the data by experts at the MHRA, which has concluded that the vaccine has met its strict standards of safety, quality and effectiveness."
Trial participants were given different dosing regimens - some received two full doses and some half a dose followed by a full dose. The MHRA has since approved the use of two full doses, which was found to be 62 per cent effective.
Prior to approval in the UK, the Oxford team reported levels of 90 per cent efficiency in participants who had received a half dose followed by a full dose of the vaccine. However, Professor Munir Pirmohamed, chair of the MHRA, said the 90 per cent efficacy rate did not hold up under analysis and that other factors may have lead to this result.
Matt Hancock said on Jan 11 that the UK has "protected more people through vaccinations than all the other countries in Europe put together".
But the vaccine roll out has not always been smooth. Some GP surgeries are being told to "pause" vaccinations in order to allow those in other areas to catch up, The Telegraph can report.

The government has promised all the over-70s, the extremely clinically vulnerable and frontline health and care workers - about 15 million people - will be offered a jab by mid-February.
However, on Jan 25, Mr Hancock admitted that supply remains "tight".
This news comes as manufacturing companies have said that they are able to produce enough vaccines, should a 24/7 roll out be introduced in the UK.
How does the AstraZeneca/Oxford vaccine work?
The vaccine – called ChAdOx1 nCoV-19 – uses a harmless, weakened version of a common virus which causes colds in chimpanzees.
Researchers have already used this technology to produce vaccines against a number of pathogens including flu, Zika and Middle East Respiratory Syndrome (Mers).
The virus is genetically modified so it is impossible for it to grow in humans.
Scientists have transferred the genetic instructions for coronavirus's specific 'spike protein' – which it needs to invade cells – to the vaccine.
When the vaccine enters cells inside the body, it uses this genetic code to produce the surface spike protein of the coronavirus.
This induces an immune response, priming the immune system to attack coronavirus if it infects the body.
How effective is it?
AstraZeneca – a British-Swedish multinational pharmaceutical and biopharmaceutical company with its headquarters in Cambridge – and Oxford University announced that their jab was effective in preventing many people getting ill and it has been shown to work in different age groups, including the elderly.
Partial immunity against Sars-Cov-2 is then detected approximately 22 days after administration of the first dose, the MHRA reported.
Data published in The Lancet in early December showed the vaccine was 62 per cent effective in preventing Covid-19 among a group of 4,440 people given two standard doses of the vaccine, when compared with 4,455 people given a placebo drug.
The overall Lancet data, which was peer-reviewed, set out full results from clinical trials of more than 20,000 people.
Among the people given the placebo drug, 10 were admitted to hospital with coronavirus, including two with severe Covid-19 which resulted in one death.
However, among those receiving the vaccine, there were no hospital admissions or severe cases.
The combined analysis from both dosing regimens resulted in an average efficacy of 70.4 per cent.
However, Deputy Chief Medical Officer Professor Jonathan Van Tam said in a press conference on Dec 30 that although people receiving the vaccine would be protected, he could not provide assurance they would not still "pose a hazard" to others in terms of passing on the virus.
"We will know quite quickly within a couple of months the impact of these vaccines on reducing severe illness in the population," he said.
"We don't know if the vaccines will reduce transmission but Public Health England have their finger on the pulse."
He would also go on to say that it would take up to two weeks for scientists to confirm the AstraZeneca and Pfizer vaccines were effective against the new strains of Covid-19.
Millions of people who have already recovered from coronavirus are likely to have protection greater than the Oxford vaccine, but, as little is known about how long-lasting immunity is, the Government continues to encourage the uptake of vaccines nonetheless.
New research from Public Health England (PHE) shows that antibodies from a previous infection provide at least 83 per cent protection from picking up the virus again, and possibly up to 99 per cent, for at least five months and probably far longer.
In contrast, the Oxford vaccine has a short-term efficacy of 73 per cent after one dose, and longer-term protection of around 70 per cent after two doses.
In a sample of more than 6,600 healthcare staff who tested positive for an infection, just 44 people were reinfected within five months, and only two of those cases were deemed "probable", with the rest being classed as only "possible".
Is it effective against the new variants?
Data on the highly contagious Covid-19 variant identified in England do not suggest that the AstraZeneca / Oxford vaccines will be less effective against it.
“We have the most data on the UK variant. That doesn’t suggest that it will be any less well protected against by the vaccine,” said Wei Shen Lim, chair of Covid-19 Immunisation on Britain’s Joint Committee on Vaccination and Immunisation, on Jan 13.
But the research is still less certain about the South African and Brazilian strain of the virus.
Oxford scientists are preparing to rapidly produce new versions of their vaccine to combat emerging Covid-19 variants from the UK, South Africa and Brazil. Although they reiterated that this should be expected, as viruses mutate all the time.
"The team do not currently think they will need to, but it would be stupid not to be prepared," a source told The Telegraph. "It should take a day or two to tweak the system."
Moderna and Pfizer-BioNTech both said their vaccines were effective against new variants of the coronavirus discovered in Britain and South Africa. But they are slightly less protective against the variant in South Africa, which may be more adept at dodging antibodies in the bloodstream.
As a precaution, Moderna has begun developing a new form of its vaccine that could be used as a booster shot against the variant in South Africa.
What has Germany said about the vaccine?
Germany is set to block the use of the Oxford coronavirus vaccine in people aged over 65 over fears it may not be effective in older age groups.
The vaccine was expected to be approved for use in the European Union on Jan 29, but a draft recommendation from Germany’s vaccine authorities called for its use to be restricted to those aged between 18 and 64.
“There is currently insufficient data to assess the effectiveness of the vaccine from 65 years of age,” the Standing Vaccine Commission (Stiko) at the Robert Koch Institute, Germany’s main centre for disease control, said.
The move comes with the European Union locked in an increasingly fraught dispute with AstraZeneca, the makers of the Oxford vaccine, and there are bound to be accusations it is politically motivated.
But the German recommendation was issued by an independent panel of doctors and scientists, who released detailed figures to back their findings.
They did not conclude the vaccine is ineffective in older people, but only that clinical trials had simply not included enough test subjects aged over 65 to provide any reliable data.
Public Health England has defended the Oxford vaccine as safe and effective for older people. Boris Johnson also jumped in to highlight that the evidence shows the jab “provides a good immune response across all age groups”, after German regulators proposed to block its use in the elderly.
Read more: German response to Oxford vaccine is unfair blow for UK scientists and will cause needless worry
How many doses does the UK have?
The UK has secured 100 million doses of the Oxford/AstraZeneca vaccine, this is the most the Government has ordered out of all of the potential vaccine candidates.
The order is enough to vaccinate 50 million people.
AstraZeneca said it aimed to supply millions of doses in the first quarter of this year as part of an agreement with the Government.
There will be four million doses available post authorisation and tens of millions of doses in the first quarter of this year.
A specific schedule is difficult to establish as batches need to be quality approved by the MHRA.
Does it differ to Pfizer and Moderna's vaccines?
Yes. The jabs from Pfizer and Moderna are messenger RNA (mRNA) vaccines.
Conventional vaccines are produced using weakened forms of the virus, but mRNAs use only the virus's genetic code.
An mRNA vaccine is injected into the body where it enters cells and tells them to create antigens.
These antigens are recognised by the immune system and prepare it to fight coronavirus.
No virus is needed to create an mRNA vaccine. This means the rate at which the vaccine can be produced is accelerated.
The Pfizer and Moderna vaccines have both been approved in the US.
The UK Government began the roll-out of the Pfizer vaccine on Dec 8.
Unlike the Pfizer vaccine, the Oxford jab does not require ultra-low temperatures.
The Oxford jab requires temperatures between 2C and 8C and can be stored for at least six months.
This is the typical temperature of a domestic refrigerator and this will make deployment of the vaccine much easier and faster.
What about antibodies and T-cells?
The Pfizer, Oxford/AstraZeneca and Moderna vaccines have been shown to provoke both an antibody and T-cell response.
Antibodies are proteins that bind to the body's foreign invaders and tell the immune system it needs to take action.
T-cells are a type of white blood cell which hunt down infected cells in the body and destroy them.
Nearly all effective vaccines induce both responses.
The Oxford/AstraZeneca vaccine induces robust antibody and T-cell responses across people of all ages, the data indicates.
Can the Oxford vaccine be manufactured to scale?
Yes. The UK Government has secured 100 million doses as part of its contract, enough for most of the population.
The head of the UK Vaccine Taskforce, venture capitalist Kate Bingham, has said she is confident it can be produced at scale.
Where is it being manufactured?
While there are some doses coming from Europe in the very first instance, the majority will be provided from the UK supply chain.
In an exclusive report on Jan 16, The Telegraph shared plans for a new £158m “super-factory”, which would produce 70m doses of an emergency vaccine on British soil- enough to vaccinate the entire nation against new coronavirus strains within four months. The factory will open later this year.
Do you need two doses of the Oxford vaccine?
The MHRA has recommended the over 18s should receive two doses to be administered with an interval of between four and 12 weeks.
When will I get my second dose?
The Government announced on Dec 30 that it was delaying the second dose of every vaccine in order to reach as many people as possible in the first round of vaccinations.
Both the Oxford vaccine and the Pfizer/BioNTech jab will be given to people as one shot, followed by another up to 12 weeks later, in order to extend some protection to as many people as possible as quickly as possible.
This is not without controversy, however.
The government's Joint Committee on Vaccination and Immunisation (JCVI) says unpublished data suggests the Oxford-AstraZeneca vaccine is still effective with doses 12 weeks apart - but Pfizer has said it has tested its vaccine's efficacy only when the two doses were given up to 21 days apart.
The World Health Organization has recommended a gap of four weeks between doses - to be extended only in exceptional circumstances to six weeks.
Will the vaccines be given out 24 hours a day?
In a further bid to accelerate vaccination, Boris Johnson has announced that 24-hour vaccine centres will be opened "as soon as we can”, with the the head of NHS England confirming on Jan 17 that several hospitals will trial 24/7 vaccine centres within the next ten days.
Matt Hancock, however, told BBC Breakfast a 24/7 approach was unlikely to be "the major factor" in hitting the mid-February target, but he was "absolutely" behind it "if it helps speed things up".
Sources in Whitehall have said that plans are in place to pilot a 24-hour vaccination centre to test demand. This comes as manufacturing companies have told ministers that they will not yet be able to produce enough vaccines should 24-hour roll out be introduced across the country.
Supplying vaccinations overnight will speed up the rollout, and allow the Government to reach their goal of vaccinating 32 million people- 60 per cent of the UK adult population by Spring-which was announced on Jan 11.
Can this vaccine help the elderly?
There have been concerns that a Covid-19 vaccine will not work as well on elderly people, much like the annual flu jab.
However, data from the Oxford/AstraZeneca trial suggests there have been "similar" immune responses among younger and older adults.
The results show that the vaccine is better tolerated in older people compared with younger adults, and produces a similar immune response in old and young adults.
Can pregnant women have the vaccine?
Pregnant women and breastfeeding mothers have now been given the green light to take either the Oxford and Pfizer coronavirus vaccines following an appropriate case-by-case risk evaluation with their healthcare practitioner.
This is a reversal of previous advice which was put in place as precautionary measure.
Traditionally pregnant women are not included in clinical trials, but following a review the MHRA are recommending pregnant women be given the opportunity to receive the vaccine as as there is no evidence they would be at risk.
Dr June Raine, chief executive of the MHRA, said: "Our advice to date has been that given that in initial lack of evidence on a precautionary basis, use of a vaccine wasn't recommended in pregnancy and women with breastfeeding should not be given the vaccine.
"But now that we have reviewed further data that has become available, the Commission on Human medicines has advised that the vaccine can be considered for use in pregnancy when the potential benefits outweigh the risks following an individual discussion with every woman."
Can people with allergies have the vaccine?
The rollout of the Pfizer vaccine was temporarily halted for those who are known to suffer from severe allergic reactions following a handful of adverse events in the initial distribution of the vaccine.
There were some concerns that this would also apply to the Oxford jab.
However, following a review, the UK regulatory body has recommended both the Pfizer and Oxford vaccine are safe to administer to those with food or medicine allergies.
Only those who have a known history of reacting to vaccines in the past should proceed with caution.
Sir Munir Pirmohamed, clinical pharmacologist and geneticist, and chairman of Commission on Human Medicine Expert Working Group, said. "We've come to the recommendation people with a known history of reacting to any specific ingredients of vaccine should not have it. But people with allergies to other medicines or food can have the vaccine."
Dr June Raine added that "at least 800,000 in the UK, probably a million and a half in the US" have already received the Pfizer vaccine.
There has been "no additional concerns and this gives us further assurance that the risk of anaphylaxis can be managed through standard clinical guidance and an observation period following vaccination of at least 15 minutes."
When will roll-out of the Oxford vaccine start?
The Oxford vaccine roll out began on Jan 4.
Mr Johnson has pledged that the NHS is committed to offering a vaccination to everyone in the top four priority groups by Feb 15.
To help with meeting this target there are already 595 GP-led sites providing vaccines with a further 180 coming on stream, he said. There are also 107 hospital sites with a further 100 to come.
Furthermore, on Jan 7, The Ministry of Defence revealed it has prepared a "reserve" taskforce of 1,500 members of the Armed Forces who are ready to work at jab centres, should the vaccinators fall ill, and extra staff are required.
The plan comes after the NHS made a formal request to the Civil Authority (Maca) convention via the Military Aid, for 133 members army personnel to take part in the vaccination programme. The workers began their training on Jan 4, and would start administering vaccines from Jan 11.
On Jan 18, a further 10 mass vaccination centres were opened in order to accelerate the roll out of the vaccines.
The Telegraph also understands the Prime Minister wants to have established 50 mass vaccination centres across England by mid-February to help drive the mass vaccination programme.
Ministers hope that the rapid expansion of these regional centres will deliver two million jabs a week by the beginning of next month.
The NHS have announced that high street pharmacies in England will also be able to distribute coronavirus vaccines from Jan 14.
Boots and Superdrug branches will be among the six stores across the country which will be able to administer the jabs.
Boots in Halifax, and Superdrug in Guildford, will be in the first group to hand out the injections, alongside Andrews Pharmacy in Macclesfield, Cullimore Chemist in Edgware, north London, Woodside Pharmacy in Telford and Appleton Village pharmacy in Widnes.
The stores have been picked because they are capable of delivering large volumes of the medicine and allow for social distancing, while still giving a spread across the country.
By the end of the month, more than 200 community chemists will be able to give vaccines, according to NHS England.
Read more: The priority list for the Oxford and Pfizer vaccines - and how they will be rolled out
Have you had the Covid vaccine or are you due to get one? We want to hear from you. Get in touch with us here.

