The pause on J&J's COVID-19 vaccine will continue for now as CDC advisors gather more information on rare blood clots
An advisory committee for the CDC endorsed keeping the hold on Johnson & Johnson's vaccine.
The vaccine was pulled after six cases of a blood clot were reported in people who got the shot.
The group of experts declined to vote on reintroducing the vaccine into the market.
An expert panel that advises the Centers for Disease Control and Prevention recommended continuing the pause on administering Johnson & Johnson's COVID-19 vaccine.
The agency is weighing whether to adjust its recommendations for the shot after at least six cases of rare but potentially fatal blood clots surfaced in people who'd recently been vaccinated.
But a last-minute advisory-committee meeting on Wednesday failed to yield a definitive answer to that question, with the panel concluding that the pause should continue and more data should be gathered.
Doctors and public-health experts overwhelmingly felt uneasy making a firm recommendation based on the minuscule amount of data available.
"I just don't feel we have enough information to make an evidence-based decision" Dr. Beth Bell, a professor of global health at the University of Washington, said during the CDC Advisory Committee on Immunization Practices (ACIP) meeting Wednesday afternoon.
"It's important from the perspective of the public: When we say rare, what does that mean? I want to be able to feel comfortable with my family members and myself getting this vaccine," she added.
More data emerging on the people with rare CVST blood clots
The CDC and Food and Drug Administration had already recommended a pause on administration of J&J's COVID-19 vaccine "out of an abundance of caution," the two agencies said in a joint statement Tuesday morning.
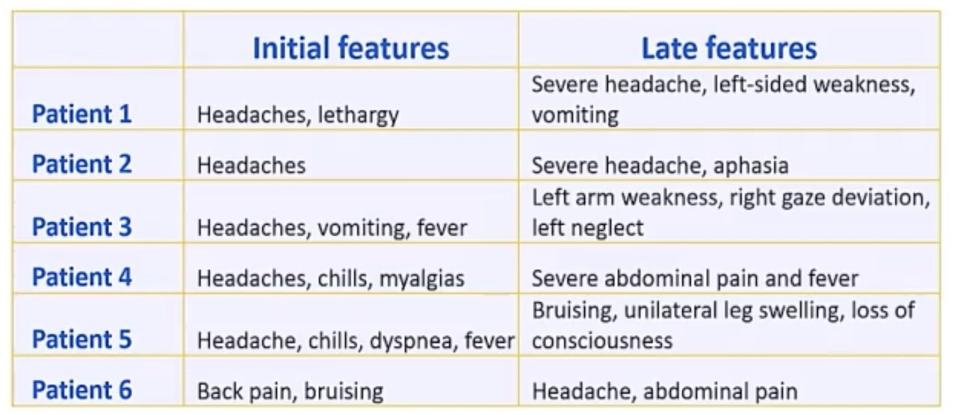
That recommendation came after at least six cases of a rare type of blood clot called cerebral venous sinus thrombosis (CVST) were reported in women across the US. The clots occurred between six and 13 days after administration of J&J's one-dose vaccine. One of the women, who was 45 years old, died, while two others are still in intensive care.
During the meeting, Aran Maree, the chief medical officer for Janssen, the medical-research arm of Johnson & Johnson, said a seventh woman, a 59-year-old, developed CVST after she received the vaccine in the US.
But it is unclear whether the clots, which are more common among young and middle-age women, are related to getting the shot. Women are already three times more likely to contract CVST than men. Symptoms usually start with some head pain.
"Really, headache is the initial presenting feature," Dr. Tom Shimabukuro from the CDC said during the ACIP meeting.
Many of the patients also had a low platelet count, which makes it difficult for the body to clot blood. A low platelet count also makes it dangerous to administer one of the most common blood-clot treatments, the blood thinner heparin.
An 'abundance of caution' prompted the pause
More than 7.2 million people across the US have received the J&J one-dose jab. It is both cheaper and easier to distribute than messenger-RNA vaccines.
With a goal to give out 200 million shots by President Joe Biden's 100th day in office (April 30) - and a global push to build immunity quickly - a pause is a setback, some of the panelists said.
"Any extension of the pause will invariably result in the fact that the most vulnerable individuals in the country who would benefit from the Johnson & Johnson vaccine will remain vulnerable," Nirav Shah, the director of Maine's CDC, said during the meeting.
The news in the US also prompted worldwide movement away from J&J's shot.

The company announced on Tuesday that it would "proactively delay" its vaccine rollout in Europe and pause all clinical trials of its vaccine around the world until guidance is updated. Australia also said it was not planning to buy any J&J shots.
South Africa is putting J&J's vaccine on pause, effectively halting that country's vaccine drive entirely, since no other COVID-19 vaccines are available there. Among the more than 289,000 healthcare workers who have been vaccinated in South Africa, none of these clots have been reported.
Tell Americans how to spot the signs of a blood clot, advisors said
One of the key reasons that the federal government recommended the pause, CDC Director Rochelle Walensky said during a White House press briefing on Wednesday morning, was to inform clinicians of the best ways to treat this type of rare clot.
"Usually, an anticoagulant drug called heparin is used to treat blood clots," the joint statement from the CDC and FDA said. "In this setting, administration of heparin may be dangerous, and alternative treatments need to be given."
There is a lot that isn't known about these blood clots and what, if any, role J&J's vaccine plays. Those open questions include how common it is for people to develop CVST blood clots when they have low platelet counts and how these clots compare with what's been reported around the AstraZeneca vaccine.
Though CVST blood clots are exceedingly rare, symptoms to watch out for include severe headache, abdominal pain, leg pain, or shortness of breath within three weeks of vaccine administration, the CDC and FDA said.
Maree said: "We strongly support ensuring vaccine awareness of the signs and symptoms of this very rare event, as well as the recommendations to ensure the correct diagnosis, treatment, and reporting by healthcare professionals."
J&J uses the same kind of vaccine technology as AstraZeneca
Last week, European regulators said potentially fatal blood clots were a "very rare" side effect of AstraZeneca's vaccine. Fifty-one of the 62 European cases reported were in women under the age of 60.
Regulators in the UK have recommended that people under 30 not take the AstraZeneca vaccine because of the very minor risk of clots, and other European countries are recommending the shots should be given to older adults only.
Both AstraZeneca and Johnson & Johnson use a viral-vector-vaccine technology, which is based on a harmless virus. The way the shot works is different from how mRNA vaccines like Pfizer and Moderna operate. That type of vaccine prompts the body to make and fight proteins from the coronavirus safely on its own.
Moderna reported 3 blood clots, but they did not come with the low platelet levels that made J&J's reports a concern
There have been at least three reported cases of CVST clotting after administration of Moderna's mRNA vaccine in the US.
But none of them have included thrombocytopenia - the low platelet level that was observed in at least four of the blood-clot patients who were administered J&J's vaccine.
Regulators have said there's no reason to suspect the three cases in Moderna recipients are related to their vaccines at all.
There have been no cases of CVST reported to regulators after administration of Pfizer's shot.
The J&J decision will not affect states that will be opening up vaccine eligibility in the coming days, officials said
The J&J decision comes as the US is pushing to offer vaccines to all adult residents by April 19.
At least five states are set to open up vaccine eligibility to all residents by Monday: Oregon, Maine, Massachusetts, Vermont, and Washington. Several other states extended residents' eligibility earlier this week.
The Biden administration has said that removing the J&J shot from the nation's vaccination article will not have a significant effect on its April 30 goal.

"Over the last few weeks, we have made available more than 25 million doses of Pfizer and Moderna each week, and in fact this week we will make available 28 million doses of these vaccines," Jeff Zients, the White House's COVID-19 response coordinator, said in a statement.
He added: "This is more than enough supply to continue the current pace of vaccinations of 3 million shots per day, and meet the President's goal of 200 million shots by his 100th day in office."
Massachusetts' COVID-19 immunization program, for one, was "built primarily on the back on the Pfizer and Moderna vaccines," Gov. Charlie Baker said Wednesday. The state received 11,600 doses of J&J's vaccine last week, compared with a combined 380,000 doses of Pfizer's and Moderna's shots.
Federal officials have already said that Massachusetts will receive about an 8% increase in its Pfizer and Moderna vaccine allocation next week, Baker said.
Massachusetts had planned to allocate its Johnson & Johnson vaccine supply to homebound patients. Replacing that vaccine with the two-shot mRNA vaccines will double the work to vaccinate those people.
Read the original article on Business Insider

