Pfizer expands recall to all lots of anti-smoking drug Chantix for carcinogen content
And now it’s four times for Pfizer — the Chantix recall, that is.
On Thursday, Sept. 16, Pfizer expanded its recall of Chantix 0.5 mg and 1 mg tablets to include all lots. In July and August, Pfizer had previously issued recalls of several lots of the popular anti-smoking medication because it might have too much of the carcinogen N-nitroso-varenicline that reaches or exceeds the U.S. Food and Drug Administration’s acceptable intake limit.
Pfizer’s latest expanded recall is meant as a precautionary measure, the company said.
Pfizer recalls another 12 lots of Chantix anti-smoking drug for a carcinogen’s presence
Chantix helps people stop smoking. It’s been recalled for carcinogen content
Reason for recall
“Long-term ingestion of N-nitroso-varenicline may be associated with a theoretical potential increased cancer risk in humans, but there is no immediate risk to patients taking this medication. The health benefits of stopping smoking outweigh the theoretical potential cancer risk from the nitrosamine impurity in varenicline,” Pfizer said in its recall notice, posted to the FDA website.
According to the FDA, nitrosamines are common in water and foods, including cured and grilled meats, dairy products and vegetables. “Everyone is exposed to some level of nitrosamines. These impurities may increase the risk of cancer if people are exposed to them above acceptable levels over long periods of time.”
Chantix is pitched to help people quit the smoking habit but it’s intended for short term use. People who smoke cigarettes are 15 to 30 times more likely to get lung cancer than people who do not smoke, according to the Centers for Disease Control and Prevention.
According to the FDA, there is no immediate risk to patients who are taking Chantix. But they ought to let their health care provider know so as to see if alternate treatments are available.
Or, well, quit cold turkey if you can.
The recalled products
The Chantix products were distributed nationwide and also to the U.S. Virgin Islands and Puerto Rico from May 2019 to September 2021.
These products include:
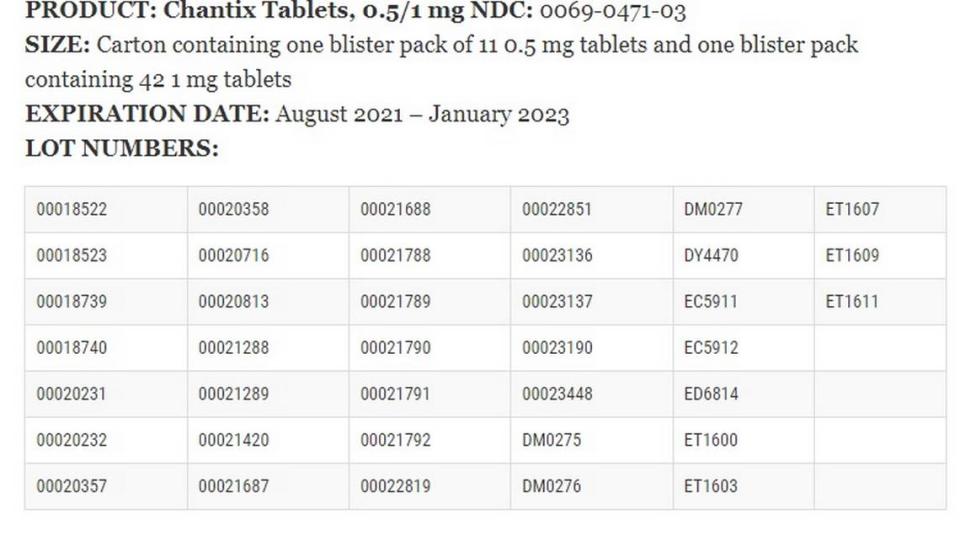
▪ Chantix Tablets, 0.5 mg NDC: 0069-0468-56 in the 56 tablet bottle size with expiration dates of January 2022 to May 2023.
▪ Chantix Tablets, 1 mg NDC: 0069-0469-56 in the 56 tablets bottle size with expiration dates of September 2021 to December 2023.
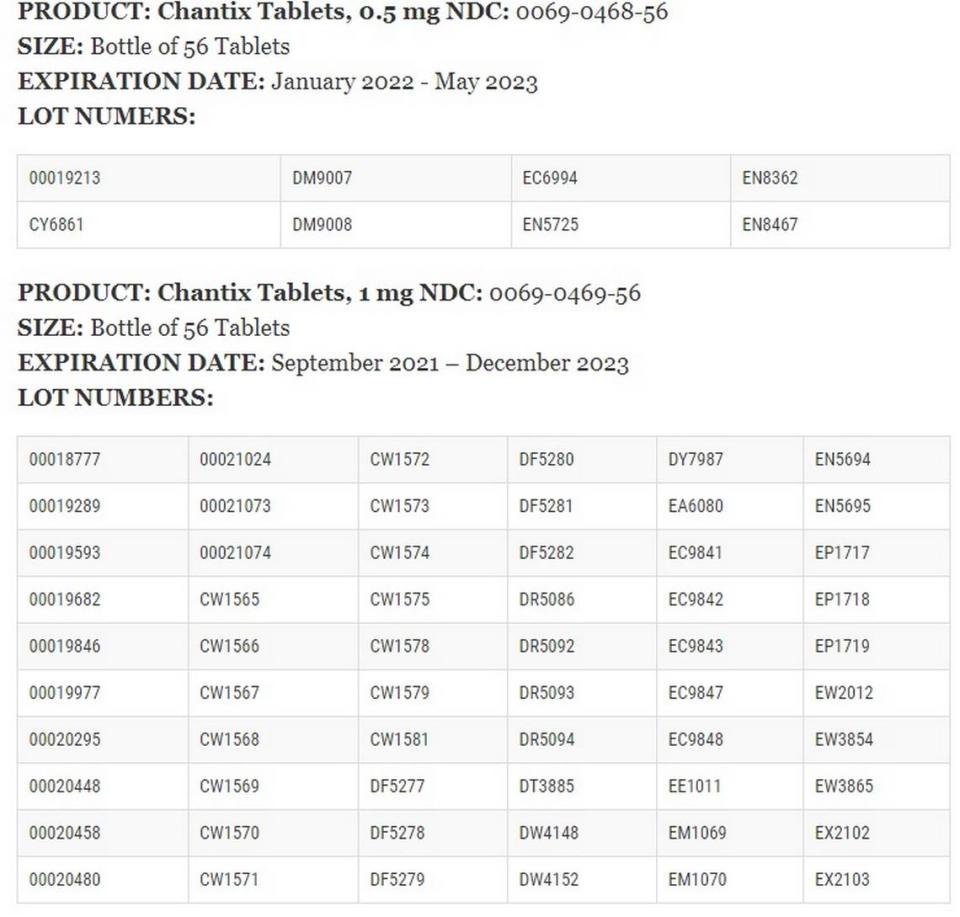
▪ Chantix Tablets, 1 mg NDC: 0069-0469-03 in the cartons containing four blister packs of 14 tablets each with expiration dates of September 2021 to June 2023.
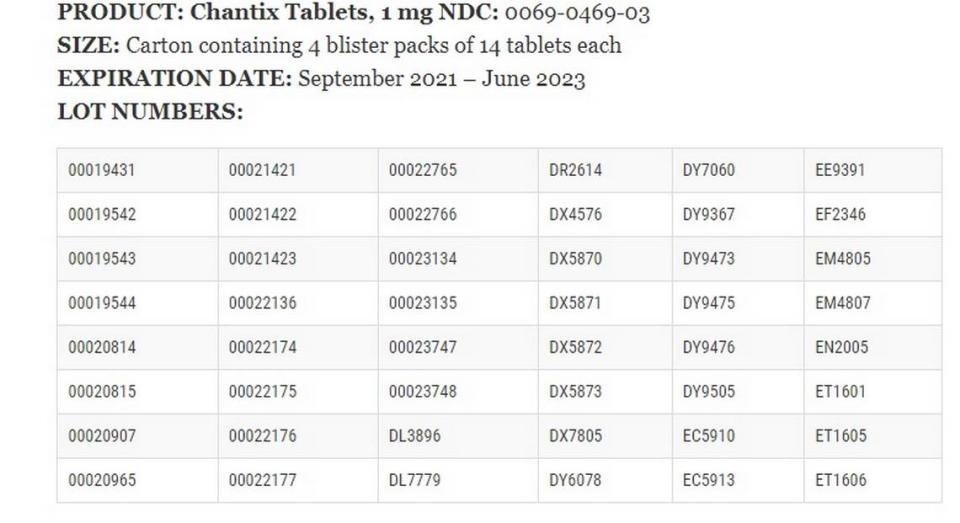
▪ Chantix Tablets, 0.5/1 mg NDC: 0069-0471-03 in the cartons containing one blister pack of 11 0.5 mg tablets and one blister pack containing 42 1 mg tablets with expirations dates of August 2021 to January 2023.
What you should do
Those who received free Chantix through Pfizer’s Patient Assistance Program or the Pfizer Institutional Patient Assistance Program, check your bottles or cartons now. If you have them, call Stericycle to return the tablets. To request replacements, call 833-203-2776, 8 a.m. to 6 p.m. Eastern time, Monday through Friday.
Wholesalers and distributors that have this inventory of Chantix tablets should stop selling or distributing them and quarantine the product immediately and notify anyone you may have distributed the Chantix to, the FDA’s alert said. You can also contact Stericycle at 888-276-6166 between 8 a.m. and 5 p.m. Eastern.

