Pharma giant Lonza begins work on another expansion at Pease campus

PORTSMOUTH — New Hampshire’s largest pharma manufacturer — Lonza Biologics — is in the process of getting larger still.
Lonza, at the forefront of vaccination efforts to combat the COVID-19 pandemic, is building onto its already large manufacturing campus, adding new manufacturing and parking on one of the last remaining vacant parcels at its Pease International Tradeport home.
On Jan. 19, the Pease Development Authority board of directors, which oversees the Tradeport, approved a revised concept plan that reduces the height of one of Lonza’s intended new buildings from three stories to one.
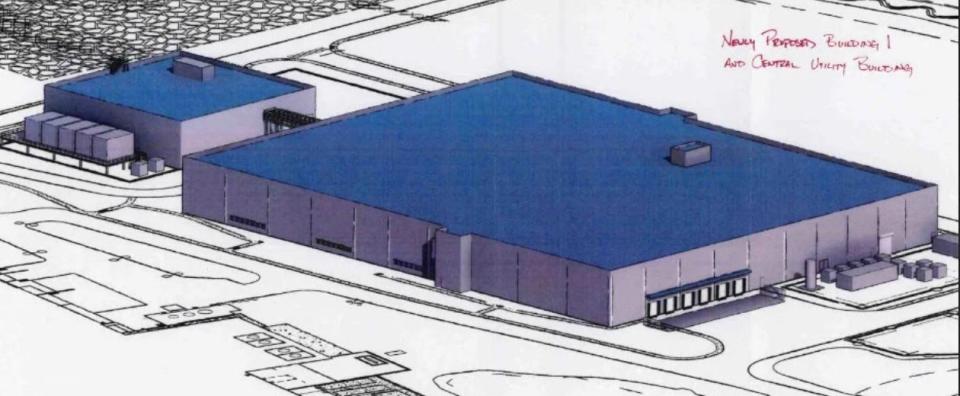
The plan relates to construction on Corporate Avenue on what has been known as the “Iron Parcel,” so named because the 23-acre hunk of undeveloped land is in the shape of an iron. Lonza has long planned to develop the parcel.
The PDA board approved a plan in 2019 for Lonza’s that included two three-story manufacturing buildings, a seven-story parking garage, central utility building, a third multi-story manufacturing building, and improvement to drainage, utilities, landscaping and traffic flow.
Child care center coming to Pease: National Guard, Portsmouth Naval Shipyard team up
The plan included a process known as “daylighting” for Hodgson Brook, which flows through the Tradeport property on its way to its outlet at North Mill Pond.
That plan was further modified recently to accommodate a client of Lonza, a contract development and manufacturing partner for biopharmaceuticals.
“Lonza is working with a client who has an immediate need for manufacturing space that can be accommodated with a one-story building,” PDA Executive Director Paul Brean said in a memo to the board. “A size reduction in Building 1 delays the need for the full buildout of the utility building and parking garage. Lonza can satisfy Building 1’s needs by constructing a utility building of about one half the size shown on the approved concept plan and by providing a temporary surface parking lot in lieu of the parking garage.”
During discussion, PDA board member Susan Parker asked if there was any way to know beforehand who the user of the space would be. “There is not,” answered Brean.
Details of Lonza's plans ‘confidential’
NHBR reached out to Lonza for comment, not only on its current expansion but whether and at what pace it continues to produce its part of the Moderna Covid-19 vaccine.
Site communications head Ela Schmuhl emailed back saying, “many aspects of the project remain confidential and we cannot comment any further on the plans beyond the publicly available information. We will reach out when details become available.”
In his memo to the board of directors, Brean noted: “Lonza will ultimately construct these facilities as approved when subsequent phases of construction are undertaken. Requiring Lonza to construct the larger facilities at this early phase would unnecessarily delay the construction and occupancy of Building 1 and could jeopardize Lonza’s commitment to its client.”
“It should be made clear that, unlike the other requested changes, the balance of Building 1 will not be completed at a later phase,” Brean added. “The building will remain as a one-story structure. Staff believes Lonza’s need to satisfy the requirements of its current client – and the opportunity to bring this biotech manufacturing to the Tradeport – justifies this change in plan.”
Lonza is a custom manufacturer for the pharmaceutical and biotech industry. It is most well-known now for making a key ingredient in the Covid-19 vaccine developed by Moderna. While the primary component of the Moderna vaccine is produced at the Lonza plant in Portsmouth, the so-called “fill-finish” work is done by Catalent Inc. in Indiana.
The Portsmouth facility is part of the Lonza Group based in Switzerland.
In additional comments sought by NHBR, Brean said, “Over the past six months it has been impressive to see sitework begin, especially the environmental initiative of the
daylighting of Hodgson Brook as it flows through the property.”
Daylighting is a process to remove obstructions (such as concrete or pavement) that covers a creek or brook, like Hodgson, and restore it to its previous original condition.
“The investment that Lonza is making at Pease provides facilities that allow them to be at the head of the spear for pharmaceutical and biotechnical development. With their partners they are developing products that will forever change healthcare. Buildings are one thing, but most importantly is the bio and pharma specific job creation on the Seacoast,” said Brean.
He added that “Lonza’s comprehensive development of the Iron Parcel at Pease will be a number of years in the making, however, this first building will result in continued creation of great career opportunities at Lonza. We’re fortunate to have a world-class entity that recognizes a unique talent pool in the area and has the ability to retain employees for long and fulfilling careers.”
These articles are being shared by partners in The Granite State News Collaborative. For more information visit collaborativenh.org.
This article originally appeared on Portsmouth Herald: Pharma giant Lonza begins work on another expansion at Pease campus

