Pharma Stock Roundup: J&J Q4 Earnings, FDA's Rejection of PFE, MRK Pipeline Drugs
This week, J&J JNJ reported mixed fourth-quarter results, beating estimates for earnings but missing the same for sales. The FDA issued complete response letters (CRL) against Merck MRK and Pfizer’s PFE regulatory applications seeking approval for their pipeline candidates, gefapixant and somatrogon, respectively. The regulatory agency approved AbbVie’s ABBV Skyrizi for active psoriatic arthritis and granted priority review to Roche’s RHHBY application seeking approval for expanded use of its spinal muscular atrophy (SMA) drug Evrysdi.
Recap of the Week’s Most Important Stories
J&J’s Mixed Q4 Earnings: J&J reported mixed fourth-quarter earnings as it beat estimates for earnings but missed the same for sales. Its Pharmaceuticals unit sales fell slightly short of expectations. Its COVID-19 vaccine generated $2.39 billion in sales for the company in 2021. The Medical Devices segment benefited from an ongoing recovery after its sales were hurt significantly in the early stages of the pandemic. However, delayed procedure volumes due to rising infection rates are hurting sales of the Medical Devices segment. Sales of the Consumer segment continued to improve.
FDA Issues CRL to Merck’s Cough Treatment: The FDA issued a CRL against Merck’s new drug application (NDA), seeking approval of gefapixant, its investigational treatment for refractory or unexplained chronic cough. The FDA has asked for more information related to the measurement of the efficacy of gefapixant in the CRL and is not related to the safety of the drug, Merck said. Gefapixant was approved in Japan last week by the name of Lyfnua tablets.
The European Commission approved Merck’s Keytruda for the adjuvant treatment of renal cell carcinoma (RCC) at increased risk of recurrence following nephrectomy (surgical removal of a kidney) or following nephrectomy and resection of metastatic lesions. This approval was based on data from the pivotal phase III KEYNOTE-564 study. The FDA had approved Keytruda for a similar indication in November.
FDA Issues CRL to Pfizer/OPKO’s Somatrogon: The FDA also issued a CRL to Pfizer and partner OPKO Health’s biologics license application (“BLA”) for somatrogon for treating pediatric growth hormone deficiency(“GHD”). In September last year, the FDA delayed its decision on somatrogon’s BLA by three months. The candidate is approved by the trade name of Ngenla in Japan, Australia and Canada.
Pfizer and partner BioNTech initiated a clinical study to evaluate an Omicron-based COVID-19 vaccine candidate. The first participants enrolled in the study have received the Omicron-based vaccine candidate as a two-dose primary series and as a booster dose. The study will include three cohorts that will evaluate different regimens of the present COVID-19 vaccine or an Omicron-based vaccine.
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) gave a positive opinion recommending conditional marketing authorization of Pfizer’s oral antiviral pill for COVID, Paxlovid. The pill has been recommended for the treatment of COVID-19 in adults who do not require supplemental oxygen and who are at increased risk for progressing to severe COVID-19. The pill was approved for emergency use in the United States in December last year.
FDA Approves AbbVie’s Skyrizi for Active Psoriatic Arthritis: The FDA granted approval to AbbVie’s Skyrizi (risankizumab-rzaa) for its second indication — active psoriatic arthritis. The approval was based on data from two pivotal phase III studies, KEEPsAKE-1 and KEEPsAKE-2. Both the pivotal studies met the primary endpoint of ACR20 at week 24. In both the studies, Skyrizi demonstrated significant improvement in joint symptoms, including swollen, tender and painful joints, compared to placebo. Skyrizi was approved for active psoriatic arthritis in Europe in November last year.
Active psoriatic arthritis is the second indication for Skyrizi in the United States. This AbbVie drug is already approved to treat plaque psoriasis.
FDA’s Priority Tag to Evrysdi sNDA: The FDA granted priority review to Roche’s supplemental new drug application (sNDA) seeking approval of Evrysdi to treat pre-symptomatic babies under two months with SMA. The sNDA application included interim data from the RAINBOWFISH study. Roche’s Evrysdi is presently approved for the treatment of SMA in adults, children and babies two months and older.
The NYSE ARCA Pharmaceutical Index declined 0.6% in the last five trading sessions.
Large Cap Pharmaceuticals Industry 5YR % Return
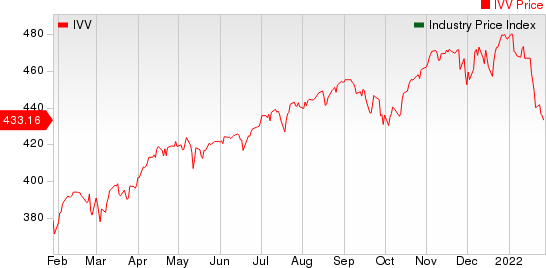
Large Cap Pharmaceuticals Industry 5YR % Return
Here’s how the eight major stocks performed in the last five trading sessions.
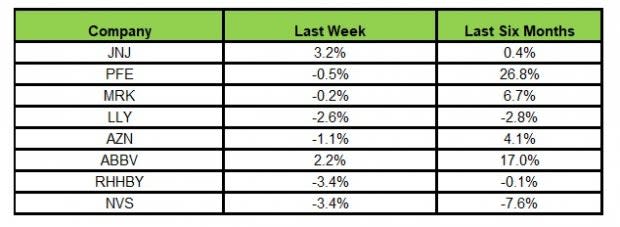
Image Source: Zacks Investment Research
In the last five trading sessions, J&J rose the most (3.2%) while Novartis and Roche declined the most (3.4%).
In the past six months, Pfizer has recorded the maximum gain (26.8%) while Novartis declined the most (7.6%)
(See the last pharma stock roundup here: GSK’s Consumer Unit Buyout Offer Rejection, FDA Updates for AZN, PFE, ABBV)
What's Next in the Pharma World?
Watch out for Merck, Lilly, AbbVie and others’ Q4 and FY21 results and regular pipeline and regulatory updates next week.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report
To read this article on Zacks.com click here.
