Philips to halt sales of sleep apnea machines in US over ongoing safety concerns
Clarification: This story has been updated to reflect the role the consent decree played in Philips' decision to stop selling CPAP or BiPAP sleep therapy devices or other respiratory care devices in the U.S.
Medical device maker Philips will stop selling sleep apnea devices in the U.S., the company announced Monday as part of a tentative agreement with federal regulators.
In 2021, the health technology company recalled millions of breathing devices and ventilators globally over concerns that foam used to reduce noise and vibration could cause health issues, including cancer.
Phillips has agreed to the terms of a consent decree preventing U.S. sales until required improvements are made at its plants, Philips disclosed in a quarterly earnings update on Monday. The deal is an effort to resolve the issues with foam in the devices that the FDA says "could potentially result in serious injury and may require medical intervention to prevent permanent injury."
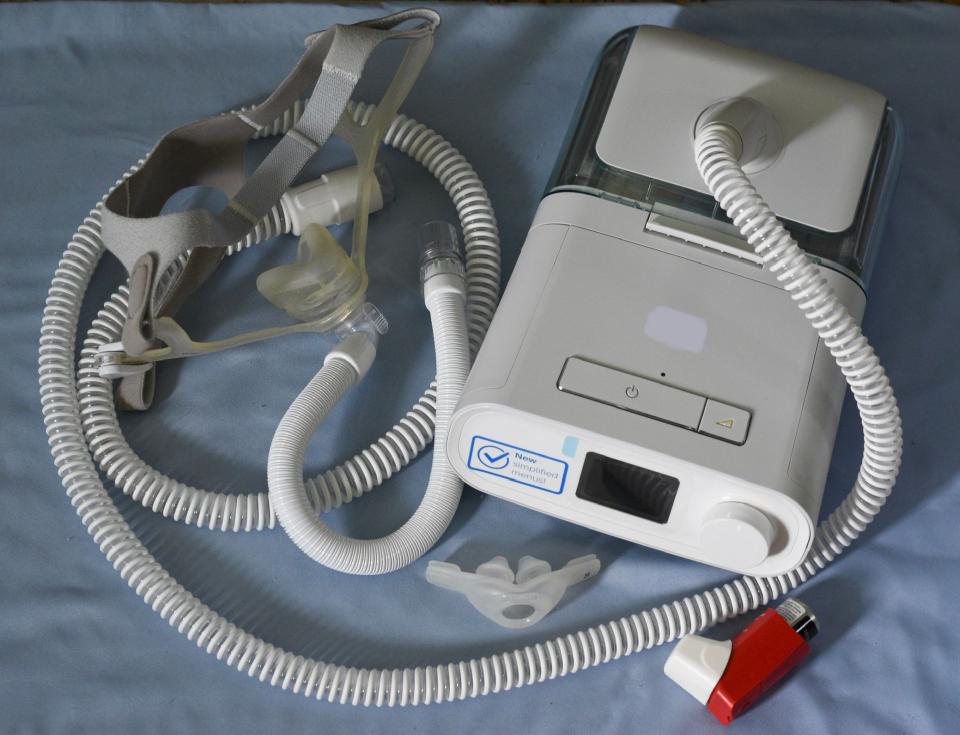
The Dutch manufacturer will continue servicing the machines already in use under the agreement with the U.S. Food and Drug Administration and Justice Department. The final amount of the settlement has not been determined but Philips has set aside about $393 million, multiple news agencies reported.
In 2022, the FDA ordered the company to improve its outreach to customers about the recall and to be more transparent about the health risks of the products. The agency estimated that only about half the Americans impacted by the recall were aware it had even happened.
Check car recalls here: Ford, Tesla, Jaguar among nearly 2.2 million vehicles recalled
Defect related to sound foam used to block noise
Philips announced a recall for millions of their Bi-Level Positive Airway Pressure (Bi-Level PAP), Continuous Positive Airway Pressure (CPAP), and mechanical ventilator devices in 2021. But "efforts to repair or replace the machines have dragged on for years, frustrating patients in the U.S. and other countries," reported The Associated Press.
The defect was related to a foam inside the devices that could degrade and cause users to breathe in particles and fumes as they sleep.
The company has said that there have been no reports of deaths, but acknowledged that the risks of particulate exposure could possibly cause "headache, irritation, inflammation, respiratory issues and possible toxic and carcinogenic effects."
The FDA’s website says that ingesting the sound-dampening foam comes with the risks of headache, asthma, allergic reactions among more serious problems. The agency also warned in November that the machines can overheat and in rare cases cause fires.
CEO promises safety and accountability
In a filing with the Securities and Exchange Commission, Royal Philips CEO Roy Jakobs promised that the company prioritizes patient safety and quality.
"Resolving the consequences of the ... recall for our patients and customers is a key focus area and I acknowledge and apologize for the distress and concern caused. We are fully committed to complying with the consent decree, which is an important step and provides a clear path forward," Jakobs said.
This article originally appeared on USA TODAY: Philips to stop US sales of sleep apnea devices over safety concerns

