Recalled contaminated eye drops linked to bacteria have led to a fourth death, CDC says
Four people have now died in a multistate outbreak of a drug-resistant bacteria strain tied to recalled eye drops, according to the Centers for Disease Control and Prevention.
The CDC and the Food and Drug Administration in February warned patients and clinicians to stop using EzriCare or Delsam Pharma’s Artificial Tears products after one death from an infection and reactions in dozens of patients, some who experienced permanent eye loss.
The number of patients treated for an extensively drug-resistant strain of Pseudomonas aeruginosa has now risen to 81 patients in 18 states – 13 more patients and two more states than in the agency's last report, the CDC said in an update issued Friday.
Seven of those patients had specimens collected after the recall's announcement and they reported use of the recalled artificial tears or lived in a long-term care facility, causing health officials' concern about potential person-to-person spread of infections. The other six cases had specimens gathered before the eye drops recall was announced. "These cases were confirmed after the recall date due to the time it takes for testing to confirm the outbreak strain and because of retrospective reporting of infections," the CDC said in the report.
In addition to the four deaths, the outbreak investigation has confirmed 14 patients with vision loss and four cases of eyeballs being surgically removed, the CDC said.
Hot car deaths The season in the US is underway. What can be done to save children?
RSV vaccine: A maternal RSV vaccine to protect infants is one step closer to FDA approval
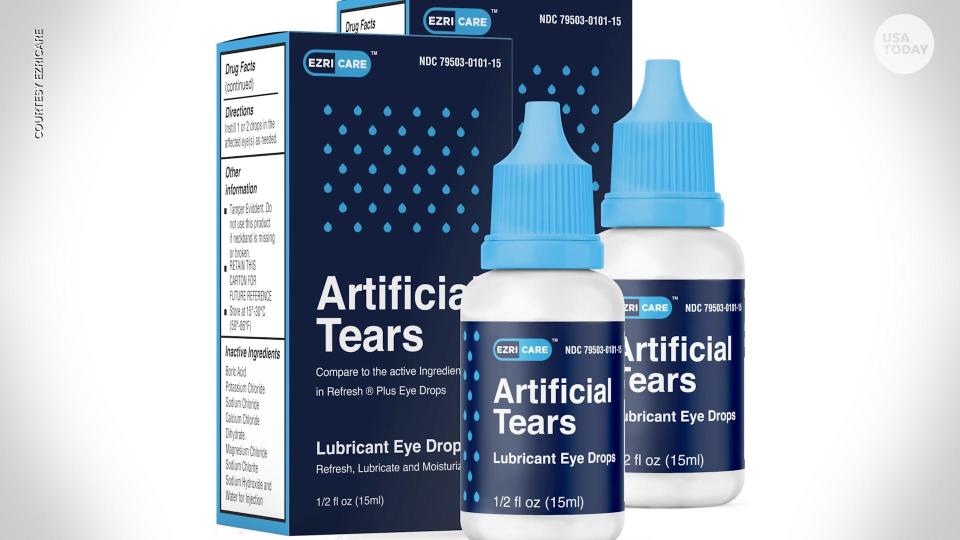
Eye drops and ointment recalled in bacteria outbreak
The products, EzriCare and Delsam Pharma’s Artificial Tears and Delsam Pharma Artificial Ointment, which were manufactured by Global Pharma Healthcare based in India, were recalled in February over potential bacterial contamination – linked to the multistate outbreak of an extensively drug-resistant strain of Pseudomonas aeruginosa.
EzriCare was the brand of eye drops used by most patients and was the only product identified in the four health care facilities where cases have arisen, the CDC says. The CDC found the bacteria strain in opened EzriCare bottles collected from patients and the FDA found contamination in unopened bottles, the agencies say.
An FDA inspection conducted after the recall found Global Pharma's production process to be unsterile: dirty equipment, faulty and ignored safety protocols and workers wearing nonsterile gowns.
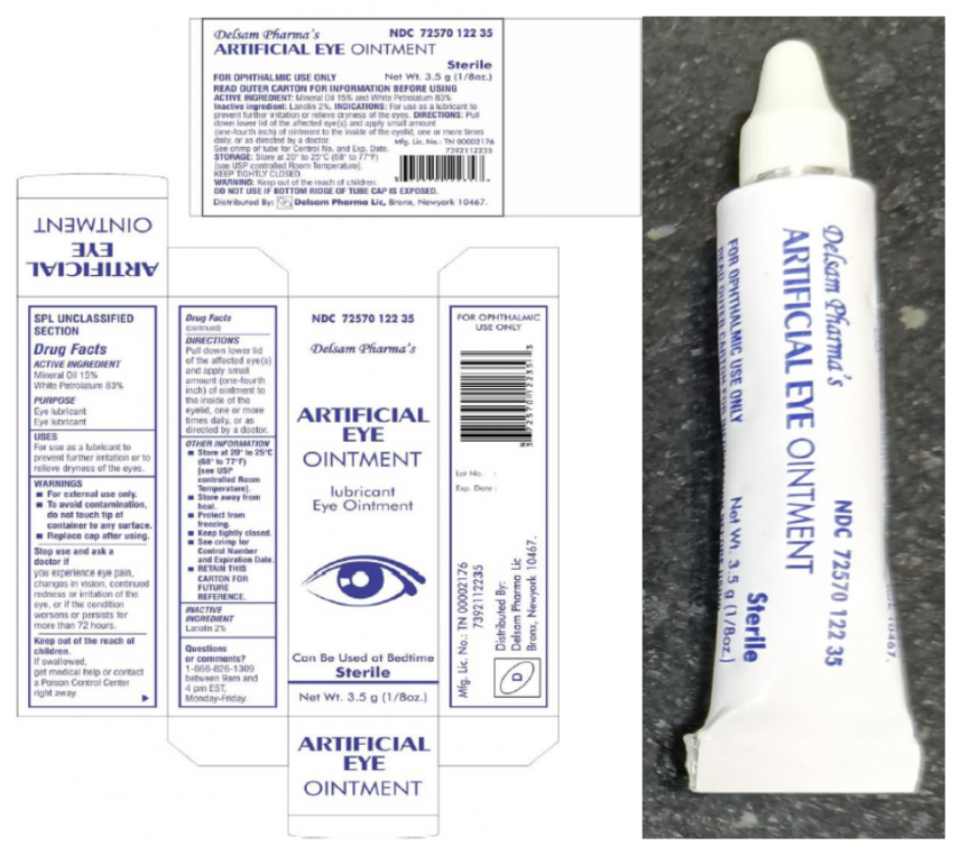
The CDC and FDA have recommended patients and clinicians should stop using and discard the products. Consumers with eye drops that are not under recall should feel safe continuing to use their products, health providers say.
Officials recommend rest homes, long-term care and other health care facilities practice infection control procedures to prevent additional person-to-person spread.
Where have patients reported eye infections during the outbreak in the U.S.?
The 18 states where cases have been confirmed by state and local health departments are California, Colorado, Connecticut, Delaware, Florida, Illinois, North Carolina, New Jersey, New Mexico, Nevada, New York, Ohio, Pennsylvania, South Dakota, Texas, Utah, Washington and Wisconsin, according to the CDC.
What is Pseudomonas aeruginosa?
There are multiple types of Pseudomonas, a bacteria that can be found in the environment including in water and soil. Pseudomonas aeruginosa causes the most infections in humans, according to the CDC.
This strain is very rare and "had never been reported in the United States prior to this outbreak," the CDC says.
Pseudomonas aeruginosa can spread to people through contaminated surfaces, equipment, water and more. The bacteria can cause infections in the lungs, blood and other parts of the body.
Pseudomonas aeruginosa "is a very dangerous bacteria because it could melt through the eye up to the cornea into the bloodstream pretty quickly," Dr. Daniel Laroche, president of Advanced Eyecare of New York and clinical associate professor of ophthalmology at Mount Sinai's Icahn School of Medicine, previously told USA TODAY.
Contributing: Wyatte Grantham-Philips
Follow Mike Snider on Twitter: @mikesnider.
What's everyone talking about? Sign up for our trending newsletter to get the latest news of the day
This article originally appeared on USA TODAY: Recalled eye drops linked to bacteria tied to four deaths, CDC says

