Regeneron (REGN) Antibody Cocktail REGEN-COV Gets WHO Recommendation
Regeneron Pharmaceuticals REGN recently announced that the World Health Organization (“WHO”) has conditionally recommended monoclonal antibody cocktail, REGEN-COV (casirivimab and imdevimab), for the treatment of high-risk non-hospitalized patients with non-severe COVID-19 and seronegative (no measurable antiviral antibodies) hospitalized patients with severe or critical COVID-19. The company’s shares declined 1.18% on Sep 24, following the announcement.
The antibody cocktail is known as REGEN-COV in the United States and Ronapreve in other countries.
The WHO Guideline Development Group reviewed data from the robust REGEN-COV development program, which reported positive phase III trial results across the spectrum of COVID-19 infection. It included the treatment of non-hospitalized patients already infected with the virus, and treatment of patients hospitalized due to COVID-19 infection, including the RECOVERY trial.
We note that Regeneron has collaborated with Roche RHHBY to increase the supply of the cocktail. Under the agreement, Roche is primarily responsible for the development and distribution of REGEN-COV outside of the United States, including pricing.
However, WHO has urged producing companies and governments to address the high price and limited production of the antibody combination from Regeneron, given the high cost and low availability of the combination therapy,
Currently, UNITAID is negotiating with Roche, which is currently manufacturing the drug for lower prices and equitable distribution across all regions, especially in low- and middle-income countries. WHO also calls for the sharing of technology to allow for the manufacturing of biosimilar versions for better access to patients in need of the same.
The company's shares have gained 32.3% in the year so far against the industry’s decline of 1.3%.
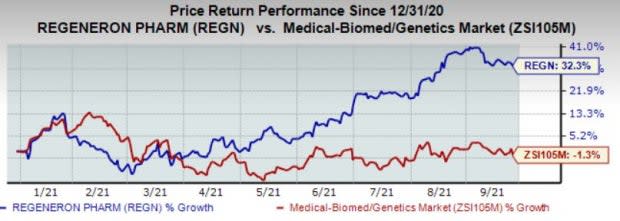
Image Source: Zacks Investment Research
Regeneron is riding high on the strong demand for REGEN-COV as the pandemic wreaks havoc with emerging deadly variants that question the efficacy of the vaccines.
Regeneron fulfilled its second agreement with the U.S. government in the second quarter to manufacture and deliver 1.25 million doses of REGEN-COV at the lowest treatment dose authorized by the FDA, and in turn, recognized $2.59 billion in REG0EN-COV sales. The company recently announced that the U.S. Department of Health and Human Services and the Department of Defense will purchase 1.4 million additional doses of REGEN-COV.
REGEN-COV was granted an Emergency Use Authorization (EUA) by the FDA for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients with positive results of direct SARS-CoV-2 viral testing, and those at high risk of progression to severe COVID-19, including hospitalization or death. In June 2021, the FDA updated the EUA by lowering the dose to 1,200 mg and permitting administration by subcutaneous injection when intravenous infusion is not feasible. The FDA also expanded the EUA to include post-exposure prophylaxis in people at high risk for progression to severe COVID-19.
The FDA has also granted an EUA to GlaxoSmithKline plc GSK and Vir Biotechnology, Inc. VIR ’s sotrovimab, an investigational single-dose monoclonal antibody, for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients and the uptake has been encouraging.
Regeneron currently sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Vir Biotechnology, Inc. (VIR) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
