Stem cells can create early human embryo structure, new study suggests
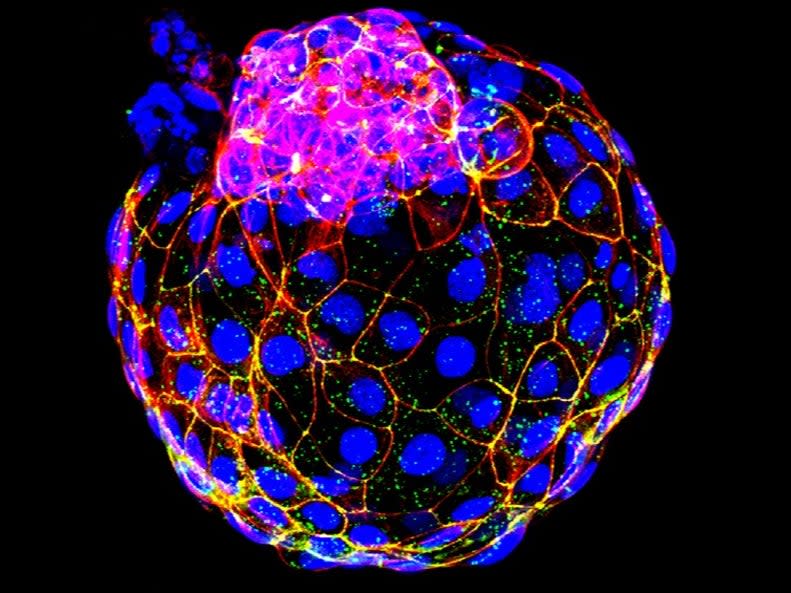
Scientists have used stem cells to accurately recreate the first stage of human embryo development, in what could be a landmark discovery for research into reproduction.
The study – published today in the journal Cell Stem Cell – saw researchers from the University of Exeter and the University of Cambridge develop a method whereby stem cells, which can turn into a number of cell types, mimic the early structure of human embryos.
Experts believe the finding could benefit research into infertility by advancing understandings of how embryos develop and the conditions needed to avoid miscarriage and other complications.
Around one in seven couples in the UK has difficulty conceiving, the experts noted.
The new embryo models can also be used to test conditions that may improve the development of embryos in assisted conception procedures such as IVF.
It comes after the discovery in March, by various research groups, that a human stem cell is able to generate the founding elements of a blastocyst, the very early formation of an embryo after a fertilised egg divides.
Professor Austin Smith, director of the University of Exeter’s Living Systems Institute, where the study took place, described the learning as revelatory.
“Finding that stem cells can create all the elements of an early embryo is a revelation,” he said. “The stem cells come from a fully-formed blastocyst, yet they are able to recreate exactly the same whole embryo structure.”
He added: “This is quite remarkable and unlocks exciting possibilities for learning about the human embryo.”
One such advancement is that scientists will no longer have to rely solely on animal research, specifically using mouse stem cells, to make judgements about human development – this is despite mice’s reproductive systems differing significantly from humans.
Access to examining human embryos is limited, and tightly regulated due to ethical considerations, but blastocysts grown in labs from human stem cells are not placed under the same ethical scrutiny, so could prove vital.
In the study, researchers arranged the stem cells into clusters and briefly introduced two molecules known to influence how cells behave in early development.
They found that 80 per cent of the clusters organised themselves after three days into structures that looked like the blastocyst stage of an embryo, a ball of around 200 cells that forms from the fertilised egg after six days.
The team went on to show that the artificial embryos have the same active genes as the natural embryo.
Dr Ge Guo, of the University of Exeter’s Living Systems Institute, said the new technique provides “for the first time, a reliable system to study early development in humans without using embryos”.
She added that the move should not be seen as a step towards producing babies in a laboratory, “but rather as an important research tool that could benefit IVF and infertility studies”.
Additional reporting by PA
Read More
Human-monkey embryo creation is worrying people: ‘This is how Planet of the Apes begins’
Scientists ‘open Pandora’s box’ with human-monkey chimeric embryo
Missouri lawmaker charged with selling fake Covid stem cell treatments
How a stem cell treatment left patients feeling worse than before

