There's a new monoclonal antibody in town: Sotrovimab is fighting omicron in Peoria
PEORIA – Monoclonal antibodies, which continue to be an effective way to reduce the severity of COVID-19 infection, have evolved over the course of the pandemic.
Just this week, the Food and Drug Administration pulled authorization on the two most-used monoclonal antibody treatments because they do not work against the omicron variant, which is now causing almost 100% of infections across the U.S.
Fortunately, there's a new formulation that does work on omicron: Sotrovimab is already being used in central Illinois.
More on the pandemic: 3 Peoria-area COVID test sites won't reopen in 'foreseeable future' due to investigation
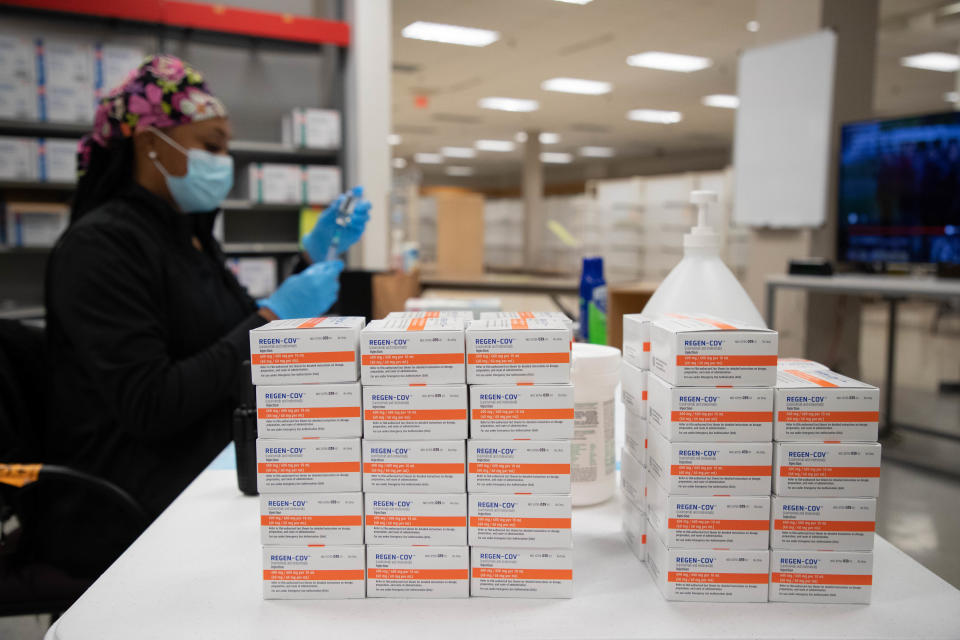
“In the last two weeks, we have shifted from Regeneron to sotrovimab,” said Dr. Shawn Piers, who oversees the monoclonal antibody infusion clinic for OSF HealthCare in central Illinois.
The process of switching
It’s not the first time health officials have had to switch gears on antibody treatments. The first monoclonal antibody treatment, which became available in central Illinois in November of 2020, was bamlanivimab, which worked well on the COVID variants circulating at that time. When the delta variant showed up, it was determined that Regeneron was more effective. Now the drug of choice is sotrovimab.
While shortages are often an issue when new treatments are introduced, they aren't the only thing pharmacists have to contend with when a new formulation comes online. Each drug must be administered in a specific way, and every time a new one is introduced, staff has to be trained on how to administer it, said Piers.
“Switching from one to the other is a process, not only for the health department and its distribution, but also in our clinics to change the process of how we give it,” he said. “Regeneron was pretty easy. It was sub-Q (subcutaneous) injection, and with sotrovimab it has to be given as an IV infusion. It is what we had to do with bamlanivimab when it first came out, so that’s not foreign us, but it is re-educating our mission partners, it is getting supplies, IV bags, tubing, poles, and developing a whole new process. So there's a lot more that goes into it when you switch from one monoclonal antibody to another.”
Not everyone got treated
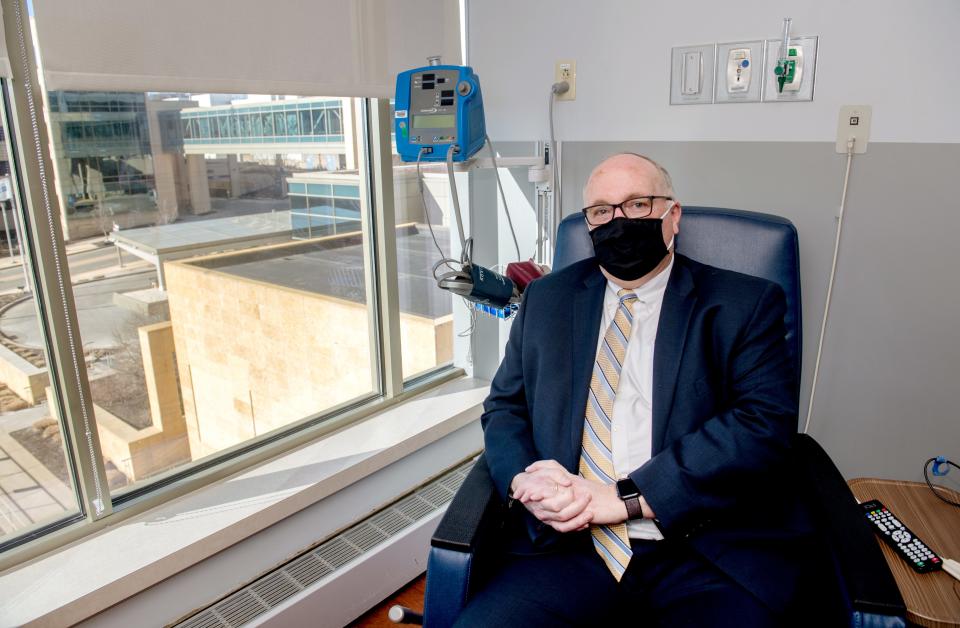
Sotrovimab takes longer to administer than Regeneron, a fact that reduces the number of patients that can be treated each day, said Piers.
"We’ve gone from treating 30 people maximally in a day to now 20 people maximally in a day because of just the time it takes to give sotrovimab to them,” he said.
Time restraints were more of an issue in previous weeks than now, since the number of people needing the treatment has gone down in recent days. For a couple of weeks, when case numbers were breaking records, there was a waiting period to receive monoclonal antibody treatments.
Omicron symptoms: How does omicron differ from earlier COVID-19 variants? We look at symptoms, risk and more
“At its peak, we were not able to treat everyone,” said Piers. “And that was even with Regeneron, not just sotrovimab. There is a priority score depending upon the risk factors that someone may have. They have to do with their age, their weight, and pregnancy is always considered high risk. Chronic kidney disease, diabetes, heart disease, hypertension, lung disease, immunosuppressive disorders or medications – those are some of the risk factors that we use to determine who is best to treat if we are running short on medication.”
Other options available
Sotrovimab, which is being distributed through state health departments, had just arrived to market during the omicron surge, and there were times when it was touch and go. But the good news is that, unlike earlier in the pandemic, there are now other options, said Alice Driscoll, regional manager of pharmacy for UnityPoint Health Central Illinois.
All hands on deck: OSF office workers train for duties at hospital, walk-in clinics
“There's a couple of oral antivirals that are now available for use, and remdesivir, which is an injectable antiviral, is also available for outpatient infusion use," she said. “We went from just having monoclonals to now having other medications in our arsenal as well, and it has helped buffer that shift from three monoclonals available to just one."
In a pinch, doctors can order an infusion of remdesivir, a drug recently authorized for outpatient use. Previously, remdesivir was reserved for hospitalized patients. It’s not being used regularly because it’s more complicated to administer, requiring three visits to the infusion clinic versus one visit for monoclonal antibodies, said Driscoll.
“We've not had to resort to that yet here in our region because we have been able to get sufficient amounts of sotrovimab,” she said.
People who become ill with COVID-19 should talk to their doctor to determine if they are a candidate for monoclonal antibodies. Time is of the essence with the treatment, which only works early in the infection. Monoclonal antibodies are only authorized for use in the 10 days after symptoms appear.
Leslie Renken can be reached at 309) 370-5087 or lrenken@pjstar.com. Follow her on Facebook.com/leslie.renken.
This article originally appeared on Journal Star: New monoclonal antibody sotrovimab now being used in Illinois

