Two recalls of ‘toxic’ hand sanitizer and over 30 additions to FDA’s Do Not Use List
Not enough alcohol, the addition of methanol, and other chemical violations ballooned the FDA’s Do Not Use List of hand sanitizers to 135 over the past week.
Also, two more hand sanitizers have been recalled and one recall’s size was clarified.
Containing methanol, which the FDA calls “toxic” in hand sanitizers, remains the main violation for hand sanitizers recalled or on the Do Not Use List. The agency clarified Monday morning that any ethanol or isopropyl alcohol with more than 630 parts per million of methanol counts as adulterated.
“Methanol exposure can result in nausea, vomiting, headache, blurred vision, permanent blindness, seizures, coma, permanent damage to the nervous system or death,” the FDA has stated. “Although people using these products on their hands are at risk for methanol poisoning, young children who accidentally swallow these products and adolescents and adults who drink these products as an alcohol (ethanol) substitute are most at risk.”
All of the eight deaths of which the FDA is aware have come from people drinking hand sanitizer with methanol, not using it as intended.
The Most Recent Recalls
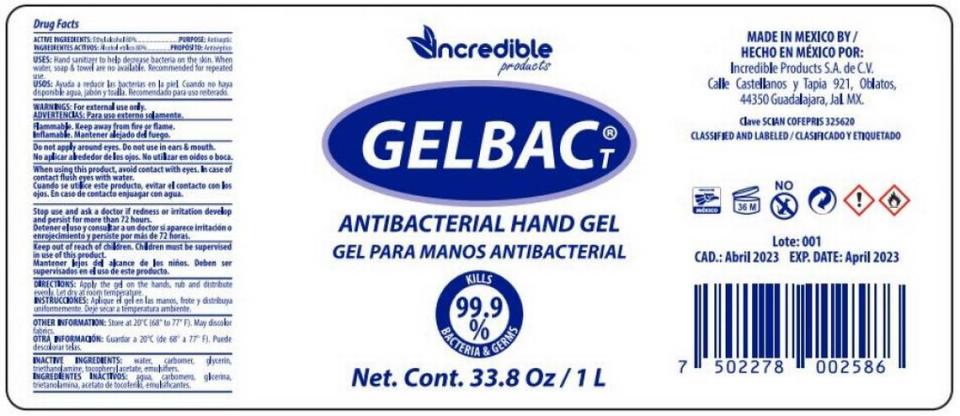
▪ Incredible Products is recalling lot No. 001 of Gelbac T Antibacterial Handgel in 4.2-ounce and 33.8-ounce bottles. The FDA found methanol in this hand santizer.
Those with questions about this recall can email gcigdl@yahoo.com.mx or dalp_07@hotmail.com.
▪ Roque Plast recalled lots Nos. 200371-12, 200371OH-05, 170420OH-06 and 170420OH-8 of Command Gel AntiBac Instant hand sanitizer after it tested positive for methanol.

Those with questions about this recall can call Rafael Cabrera + 52 55 58165506 Ext. 103, Monday through Friday, 10 a.m. to 5:30 p.m., Eastern time or email antibac@roqueplast.com.
▪ Soluciones Cosméticas was among the first to recall its hand sanitizer when it pulled all lots of 16.9-ounce bottles of Bersih Hand Sanitizer Gel Fragrance Free in July. But, Sunday, it clarified that it meant all lots, both the bottles with the blue tops (mentioned in the original recall) and the bottles with the green tops, with UPC Codes 816822026667 or 7503007103178. The lot numbers recalled are 0100K01 to 0148K01.

Consumers with questions about this recall can call 866-912-8410, Monday through Friday, 8 a.m. to 5 p.m., Eastern time, or can email bersih bersihrecall6551@stericycle.com.
The FDA’s Do Not Use List additions
▪ Asiaticon’s Astrum-distributed V-KLEAN Hand Sanitizer Gel tested as being too light in the ethanol alcohol content.
On the list because they were reported to be made in the same location are Asiaticon, Acadia Mercantil, and Protex Labs-distributed V-KLEAN Hand Sanitizer Gel; Derma 70 Hand Sanitizer; SWCH-distributed V-KLEAN, Medically Minded Hand Sanitizer Gel and Protz Real Protection Antibacterial Hand Sanitizer.
▪ Botanicals Internacional’s All Clear Hand Sanitizer Fragrance Free, distributed by Good Fibers, tested as having methanol, but the FDA says it hasn’t been seen on U.S. shelves.
Also on the list because they’re believed to be made at the same facility are Pure Haven-distributed Alcohol Antiseptic 80% topical solution hand sanitizer; Inatek Hand Sanitizer Non-Sterile Solution; Total Pure Alcohol Based Hand Sanitizer Gel; USMed Supplies distributed 70%Alcohol Gel Hand Sanitizer; ResQue 1st Instant Hand Sanitizer; and LTD Enterprises-distributed 70% Alcohol Gel Hand Sanitizer.
▪ DEPQ International’s dgreen Advanced Sanitizer Alcohol Free didn’t test as having methanol, but its “benzalkonium chloride level was subpotent.”
Benzalkonium chloride-based hand sanitizers, as opposed to alcohol-based hand sanitizers, aren’t recommended by the CDC, but FDA standards allow them to be sold in the United States.
Clean Humans Hand Sanitizer, dgreen Advanced Hand Sanitizer Antibacterial Gel and Biokaab-distributed Hand Sanitizer Gel all made the list out of association, “purported to be made at the same facility.”
▪ Estrategia Hospitalaria’s OZO Hand Sanitizer, Luxury Formula had methanol and an ethanol alcohol level that was too low, according to the FDA. The agency also said it hears that Ismar Soluciones Dinamicas’s OZO Hand Sanitizer, distributed by Ancorp Capital Group, is made at the same place.
▪ Grupo Insoma, which already had one hand sanitizer on the list, now has one distributed by CIRG Waste & Recycling Solutions US. It doesn’t have a National Drug Code number.
▪ Notarika’s Greenfrog Hand Sanitizer tested as having methanol, the FDA says, so that and Greenfrog Hand Sanitizing Wipes are on the list.
▪ The FDA says Noticas Mexico Hoy Grupo Multimedia’s Medical Mary Clean Hand Sanitizer tested as having methanol, but the agency “has been unable to contact the manufacturer” to ask for a recall of Medical Mary or AMX Instant Hand Sanitizer.
▪ Quimica Magna de Mexico’s Datsen Hand Sanitizer and Alcohol Antiseptic 62% Hand Sanitizer tested as subpotent, the FDA said. The CDC recommends at least 60% alcohol content for hand sanitizer).
Believed to be made at the same place are Bernal Hand Sanitizer, Alcohol Antiseptic 65% Hand Sanitizer and Alcohol Antiseptic 70% Hand Sanitizer, distributed by Inflatables.
▪ Spartan Chemical out of Ohio found microbial contamination in its Lite ‘n’ Foamy Lemon Blossom Hand Sanitizer and foamyIQ Lemon Blossom Hand Sanitizer. Both were recalled July 1.
Here’s why a mildew stain removal spray sold at Publix and elsewhere got recalled
The onion salmonella outbreak grows. More recalls from Walmart, Kroger, Publix, H-E-B
There are more problems with onions. Another brand pulls products at Walmart and Kroger

