US to release at least 1.6 million vaccines to fight monkeypox by end of year, officials say
More than 1.6 million vaccines to combat monkeypox will be released in the United States throughout the rest of the year, and anyone possibly exposed to the virus is encouraged to get vaccinated, federal health officials said Tuesday.
The U.S. Department of Health and Human Services will release 56,000 doses of the Jynneos vaccine immediately in areas where monkeypox transmission rates are high, followed by an additional 240,000 doses in the coming weeks. The vaccines will be distributed through a tier system, prioritizing areas with a high number of confirmed cases.
The move comes as the U.S. has recorded more than 300 cases of monkeypox across more than two dozen states.
"We are recommending that vaccines be provided to both people with known monkeypox exposures who are contacted by public health, and also to those people who've been recently exposed to monkeypox but may not be identified through case investigation and contact tracing," Centers for Disease Control and Prevention Director Rochelle Walensky said Tuesday.
Jennifer McQuiston, deputy director of CDC’s Division of High Consequence Pathogens and Pathology, said intimate or sexual contact "seems to be a primary driver for transmission."
Health officials said those who have had a sexual partner diagnosed with monkeypox, as well as men who have had sex with other men who have had multiple sexual partners in areas where monkeypox cases are rising, should get vaccinated.
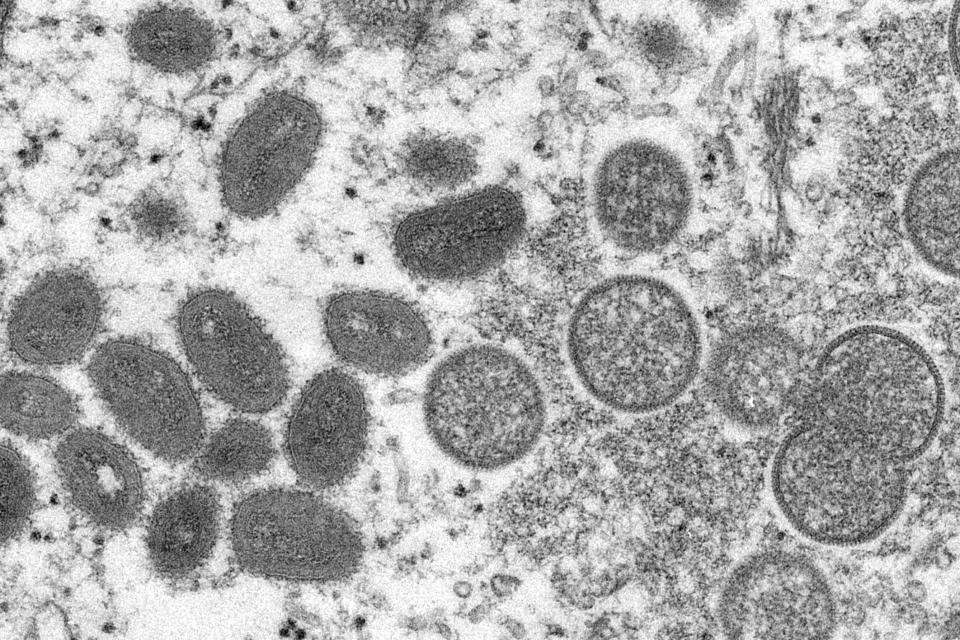
There are no treatments specifically for monkeypox infections, but smallpox viruses are genetically similar, meaning smallpox vaccines could be used to prevent monkeypox infections. The Jynneos vaccine is one of those vaccines, and it was approved by the Food and Drug Administration in 2019 for monkeypox prevention in people ages 18 and older. It requires two doses, taken four weeks apart.
Research data from Johns Hopkins Center for Health Security suggests the vaccine is 85% effective against preventing monkeypox, but it is unknown how well it protects humans from contracting the virus.
On top of the nearly 300,000 vaccine doses becoming available in the U.S. in the next few weeks, the HHS expects 750,000 additional Jynneos vaccines will be made available over the summer, with 500,000 doses released throughout the fall, pushing the expected total to 1.6 million doses this year.
Monkeypox vaccines: What to know about the Jynneos vaccine
Closer look: Rising reports of rare monkeypox cases in US and around the world raise concern
The announcement comes the same day the CDC announced it was activating its Emergency Operations Center, which "allows the agency to further increase operational support for the response to meet the outbreak’s evolving challenges."
'This is not a novel virus'
The concern surrounding the rise of monkeypox, which is typically found in parts of Central and West Africa, comes because cases have popped up in places where it usually isn't reported. More than 4,700 cases have been reported in 49 countries this year as of Tuesday, Walensky said. The largest outbreak is in the United Kingdom.
In the U.S., 306 cases have been reported in more than 26 states, most of them in California, New York, Illinois and Florida, according to CDC data.
Caused by a virus in the same family as smallpox, monkeypox is transmissible through person-to-person contact with rashes, scabs or bodily fluids, as well as touching infected items such as clothes. Symptoms, which can begin to appear seven to 14 days after exposure, include fever, muscle aches, exhaustion and a rash that can appear through the body. It is fatal for up to 1 in 10 people, the World Health Organization says.
Areas where monkeypox transmission is high can also request shipments of the ACAM2000 vaccine. Although the vaccine has proved effective against the virus, the FDA says, it carries a risk of serious side effects, such as myocarditis and pericarditis, or inflammation and swelling of the heart and surrounding tissues.
White House COVID-19 response coordinator Dr. Ashish Jha said monkeypox outbreaks have happened before and have been handled effectively.
"This is not a novel virus. Unlike COVID of two years ago, monkeypox is a virus that's been around forever. We've known about it for at least 60-some-odd years, and we've spent years studying and treating monkeypox in endemic nations," Jha said. "This outbreak requires both vigilance, as well as a comprehensive approach, so that's why we're prepared to act rapidly."
Contributing: Mike Snider, Elizabeth Weise
Follow Jordan Mendoza on Twitter: @jordan_mendoza5.
This article originally appeared on USA TODAY: Monkeypox outbreak: 1.6 million vaccines to be released in the U.S.

