What it was like to volunteer for the J&J vaccine trial in Chile
By Aislinn Laing
SANTIAGO (Reuters) - The idea to sign up for the trial of a COVID-19 vaccine developed by Johnson & Johnson came to me after interviewing the company's Latin American vice president for medical affairs in September.
Josue Bacaltchuk told me that volunteering for trials meant being part of the solution to a global problem, and the media could play a critical role in scrutinizing vaccine development and building confidence among people who might receive them.
All of us, these past nine months, will have felt at times overwhelmed by the sadness generated by this pandemic.
To this day, my heart contracts when I see signs telling people: "Keep your distance" and "Stay apart" - it seems so contrary to human nature.
As a family, we have always trusted medical science and so far that trust has been rewarded. Our three little boys' vaccine schedules are up to date and they are hale and hearty. My husband successfully beat back testicular cancer after chemotherapy and great medical care.
My mother and my mother-in-law are both at high risk of getting seriously ill if they contract COVID-19, and have been shut inside for long spells, as has my 98-year-old grandmother, who is sociable and loves doing her own shopping.
Participating in the trial was simple: I filled in an online form, then a nurse from the local Janssen trial team got in touch and asked me to fill out a more detailed one. I was then invited to Colina, a scruffy town to the dry and dusty north of Santiago, to be screened.
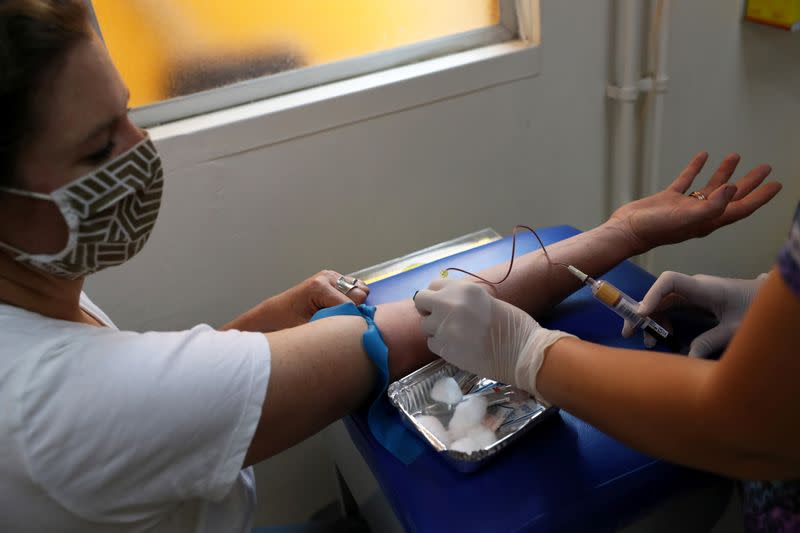
I gave some vials of blood to be tested for antibodies to see if I had already had the virus, took a pregnancy test, and underwent a deeply unpleasant nasal swab for a COVID-19 PCR diagnostic test.
The medical teams were busy but clearly excited to be part of something so important. The sentiment was contagious and volunteers filing in and out of the shipping container acting as the trial's nerve center grinned at each other and gave the thumbs up.
A doctor sought to determine my medical history and risk of catching the coronavirus. Did I work in an office? How did I travel there? Were my children in school and when was the last gathering I attended?
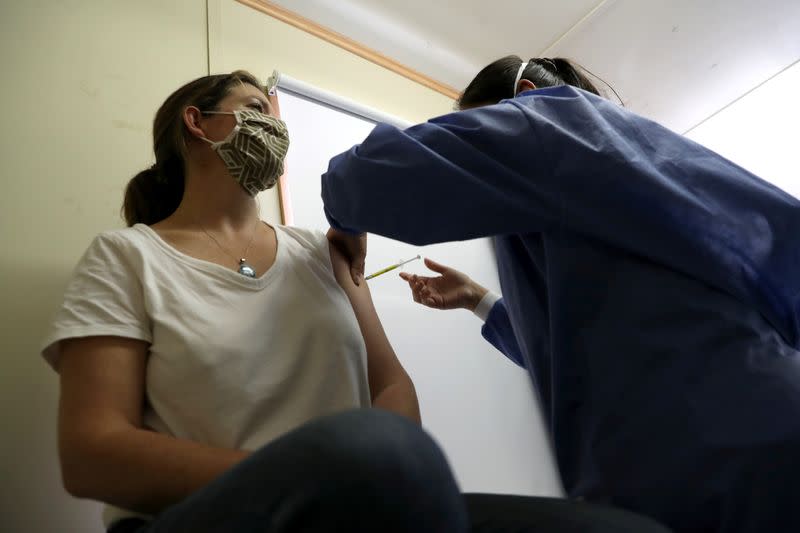
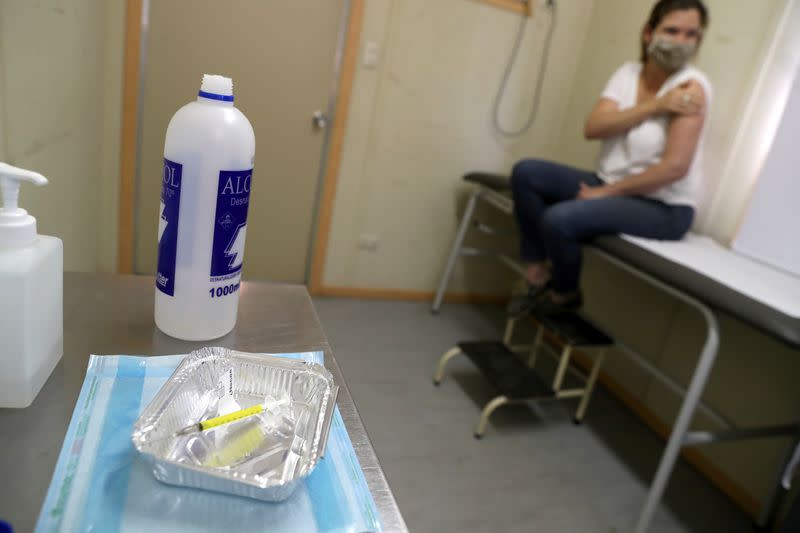
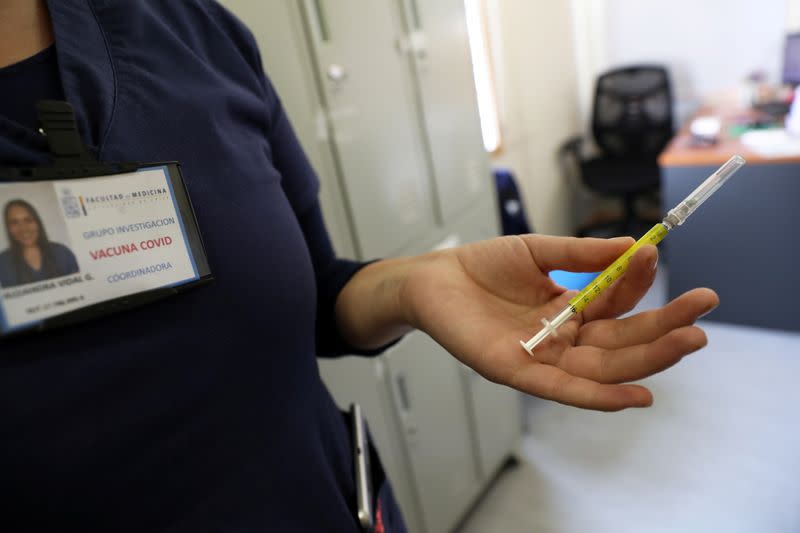
I passed the tests and was ushered into another shipping container for my injection. It was over in minutes. I had a 50-50 chance of getting the actual vaccine or a saline solution placebo in the double-blind trial. I was monitored for a few minutes to make sure I had no immediate reaction, then thanked and sent home, with only a tiny circular plaster to show for my brush with a product the world is talking about.
That evening, I experienced a banging headache, a sore jaw and a bit of nausea, although of course I don't know if that was caused by the vaccine or something else. I went to bed early and woke up feeling fine.
This trial is testing a single-dose vaccine, potentially a selling point if the data is good and it's approved.
I returned to the trial site last week to give more blood. That will allow the investigators to see if I developed antibodies to combat the virus or because I contracted COVID-19 unawares, and if the vaccine they may have given me helped diminish its impact.
(Reporting by Aislinn Laing, Editing by Rosalba O'Brien)