NJ Lyme disease cases rising. Could this vaccine kill it for good?
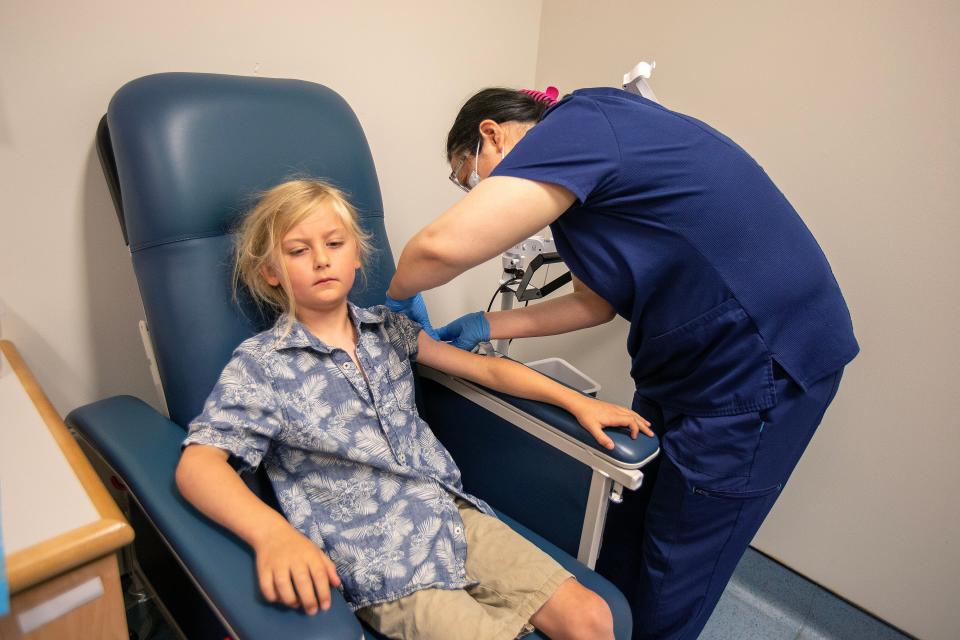
NEW BRUNSWICK - Seamus Naughton Mergner, 7, hopped into an exam chair at Robert Wood Johnson University Hospital one morning last week, a perfect candidate for the clinical trial underway for a Lyme disease vaccine.
Seamus lives in a home that sits on five acres in woodsy Allentown, where ticks would flourish. He's had the illness, so his family understand the threat. And, maybe most importantly, he isn't afraid of needles.
"Lyme is definitely more of a concern to me than the vaccines," said Seamus' mother, Taylor Naughton. "The idea of contributing to an effective vaccine, I'm like, 'Yeah, let's do that.'"
Researchers are in the final stages of clinical trials to develop a vaccine that would prevent Lyme disease, helping people who live in high-risk areas avoid an illness that can be debilitating.
'I was not giving up on this dream': Lyme disease was brutal, but she kept fighting to become a doctor
The potential breakthrough would be timely. Health experts say the population of ticks, which transmit the disease, is on the rise. And the clinical trial appears to have found enough volunteers like Seamus, overcoming an anti-vaccination sentiment that seemed to grow louder during the COVID-19 pandemic.
"This disease can be debilitating in some people, and it can last a long time," said Dr. Sunanda Gaur, director of the Pediatric Clinical Research Center at Rutgers Robert Wood Johnson Medical School.
How long before the vaccine is available?
Seamus Naughton Mergner had few words — well, no words, really — before he got his second shot last week. He sat through it without a wince, before hopping out of the chair to give his mother a comforting hug.

Rutgers wants your tick pics: Research to help monitor tick-borne disease spread in NJ
The shot was either the vaccine or a placebo — it's a blind study, so Seamus and his family don't know which it was. But Seamus didn't have any side effects after receiving his first dose in April. In all, he and other participants will receive three doses, followed by a booster shot.
The vaccine is being developed by Pfizer and Valneva, a French company that researches specialty vaccines. It is the only active Lyme disease vaccine in a clinical trial. And the trial is in its third stage, the final step before it goes to the U.S. Food and Drug Administration for review.
Rutgers Robert Wood Johnson Medical School is one of about 50 sites participating in this stage of the trial, The sites have recruited some 3,000 volunteers ages 5 and older who live in regions in the U.S. and Europe where Lyme disease is found regularly.
The trial hasn't been without missteps. In February, the two companies said a study with about half of the participants was being discontinued because a third-party contractor didn't follow clinical guidelines. The companies said they hoped to apply for regulatory approval in 2026.
Still, analysts think the demand for a Lyme disease vaccine would be high.
"Obviously we have to await the results of the trial, but assuming that (the vaccine is) going to be effective, I think it's an important step forward," said Dr. Peter Krause, senior research scientist at the Yale School of Public Health in New Haven, Connecticut. The vaccine "wouldn't be for everyone, but in people who are at risk, I think it definitely makes sense."
Tick Act passed by Congress: How much of $150M goes to Lyme disease research?
How do you get Lyme disease?
The illness is transmitted to humans by ticks, parasites as small as a poppy seed that need blood to survive, The ticks become infected with Lyme disease bacteria feeding on wildlife, usually rodents. And they pass the bacteria along during their next stage of life, according to the U.S. Centers for Disease Control and Prevention.
While deer are important hosts for ticks, they don't carry the Lyme disease bacteria, the CDC said.
In most cases, a tick needs to be attached to people for 36 to 48 hours to transmit the disease. Once exposed, people often have a bull's-eye rash along with other symptoms such as fever, chills, headache, fatigue, muscle and joint aches. The illness is commonly treated with antibiotics, but more symptoms can last for months: severe headaches, severe joint pain, facial palsy, irregular heartbeat and nerve pain.
New Jersey has averaged about 3,500 cases between 2001 and 2020, with Morris and Monmouth counties reporting the most incidents, according to CDC data, although the agency says the disease is vastly under-reported.
Despite reporting discrepancies, the Garden State has routinely had among the most cases nationwide, CDC data shows, and cases have been rising for the past two decades, from a low of 2,017 in 2001 to a peak of 5,091 in 2017.
Among the reasons for the increase: Climate change, which has made the environment more hospitable for ticks, and encroaching development in rural areas, experts said.
Fighting the ticks: One way to get rid of disease-carrying ticks in the Pine Barrens? Burn 'em
How is Lyme disease treated?
Caught early enough, Yale's Krause said, Lyme disease can be treated successfully with antibiotics. But some patients continue to have long-lasting symptoms, prompting drugmakers to continue their research for a vaccine.
The Pfizer-Valneva vaccine wouldn't be the first for Lyme disease.
SmithKline Beecham developed a Lyme disease vaccine that was approved by the FDA in 1998. But it ran into obstacles: It required three shots along with a booster; it was found to be just 75% effective; and recipients sued the drugmaker after they developed arthritis, Krause said.
Studies found no link between the vaccine and arthritis, Krause said, but the company's successor, GlaxoSmithKline, removed the vaccine from the market because of plummeting sales.
"The arthritis was not due to the vaccine; it was due to genetic components in your body that led to that," he said. "Obviously if you've recently gotten vaccinated and then you develop arthritis, you think it's through the vaccine. In fact, the data showed that wasn't the case."
Will New Jerseyans line up for the shots?
With the Pfizer and Valneva trial underway, the anti-vaccine campaign has seemingly grown stronger. Even before the COVID-19 vaccine turned into a political battle, the anti-vaccination movement made inroads that led to the comeback of diseases once were thought to be eliminated, researchers said.
Still, public sentiment isn't clear-cut. Some 88% of Americans say the benefits for children of the measles, mumps and rubella vaccine outweigh the risks, similar to the percentage in 2019, according to a Pew Research Center survey released in May.
But nearly half of those with a young child under age 4 say the statement “I worry that not all of the childhood vaccines are necessary” describes their views at least somewhat well, the study found.
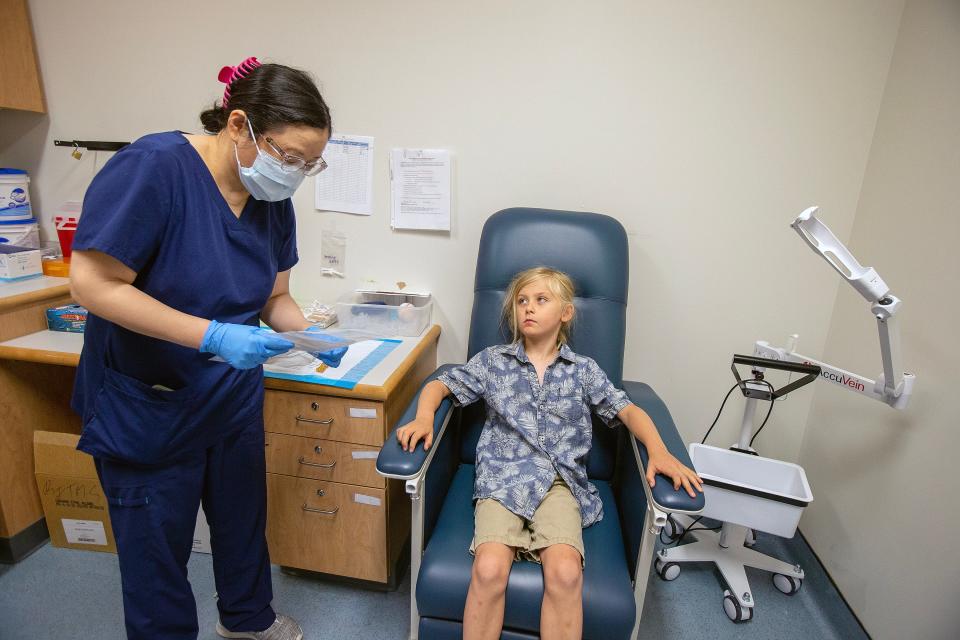
During an interview in early May, Gaur of Rutgers said she worried about finding enough volunteers for the Lyme disease study. But by the time Seamus Naughton Mergner arrived for his second dose last week, the clinic had found enough candidates and cut off enrollment.
"If you look at most of the studies with vaccine hesitancy, with COVID in particular in children, the parents are most likely to change their mind or follow the lead of the pediatrician," Gaur said. "So we have a very important role to play despite all the issues. So we keep doing it, we keep working at it."

Taylor Naughton didn't need much convincing. She was scrolling through her Instagram page one day last spring when she saw an advertisement seeking children to participate in a clinical trial for a Lyme disease vaccine.
Naughton was interested. She once had Lyme disease. Seamus had already had it. And, she said, her sister had the disease that initially went undetected and caused chronic problems. When Naughton asked her son if he wanted to participate, he decided it would be a good idea.
"I think I just asked him if he wanted to do it so he could potentially be protected from getting Lyme, and yeah, he was fine with that," Naughton said.
Michael L. Diamond is a business reporter who has been writing about the New Jersey economy and health care industry for more than 20 years. He can be reached at mdiamond@gannettnj.com.
This article originally appeared on Asbury Park Press: Lyme disease vaccine from Pfizer, Valneva tested on NJ children

