Walmart brand and others begin the recalls of eye drops made in ‘insanitary conditions’
An FDA warning about six brands of eye drops made in “insanitary conditions” was backed up Tuesday as one brand said its users have reported “vision blurriness, vision loss and burning eyes.”
That’s from The Harvard Group’s recall notice about two kinds of eye drops sold under the Rugby brand. The same day, Cardinal Health also recalled six kinds of Leader brand eye drops. And Walmart yanked two lots of Equate eye drops on Monday.
What does Velocity Pharma have to do with these eye drops?
What the recalled eye drops and the other eye drops in the FDA’s original warning — sold under Velocity Pharma, Target’s Up&Up brand, CVS and Rite Aid store brands — have in common is being manufactured in India by Velocity Pharma.
The FDA said it recommended “the manufacturer of these products recall all lots on Oct. 25, 2023, after agency investigators found insanitary conditions in the manufacturing facility and positive bacterial test results from environmental sampling of critical drug production areas in the facility.
“These products are intended to be sterile,” the warning explained. “Ophthalmic drug products pose a potential heightened risk of harm to users because drugs applied to the eyes bypass some of the body’s natural defenses.”
If you have health problems from these eye drops, first, tell a medical professional. Then let the FDA know via a MedWatch report, which can be done online or by calling 800-332-1088 to get a pre-addressed form that can be mailed or faxed to 800-332-0178.
After that, call the company involved.
What eye drops have been recalled?
▪ Walmart pulled lot Nos. KRPE 3090 and KRPE 3091 of Equate Hydration PF Lubricating Eye Drops (Propylene Glycol Eye Drops 0.6% w/v), UPC code No. 00194346058815. They were first sent to Walmart stores on Sept. 1.
Throw the eye drops away or return them to the store for a refund. Walmart is asking for a receipt, but seeing as how this is their store brand, there’s not much doubt about who you paid for the eye drops. If you have questions about this recall, contact Walmart either online or by calling 888-287-1915, Monday through Friday, 9 a.m. to 6 p.m, Eastern time.
▪ Cardinal Health recalled all lots of six kinds of eye drops sold under the Leader brand:
Eye Irritation Relief (Polyvinyl Alcohol,0.5%, Povidone, 0.6%, andTetrahydrozoline Hydrochloride, 0.05%) in 15 ml bottles; Dry Eye Relief (Carboxymethylcellulose Sodium, 1%) in 15 ml bottles; Lubricant Eye Drops (Carboxymethylcellulose Sodium, 0.5%) in 15 ml bottles, sold alone and in pairs; Lubricant Eye Drops (Propylene Glycol, 0.6%) sold in 10 ml bottles; Dry Eye Relief (Polyethylene Glycol 400,0.4% and Propylene Glycol, 0.3%) in 10 ml bottles.
Cardinal said it knows of three users who suffered health problems after using the eye drops. They’ve been distributed nationwide since Dec. 12, 2021.
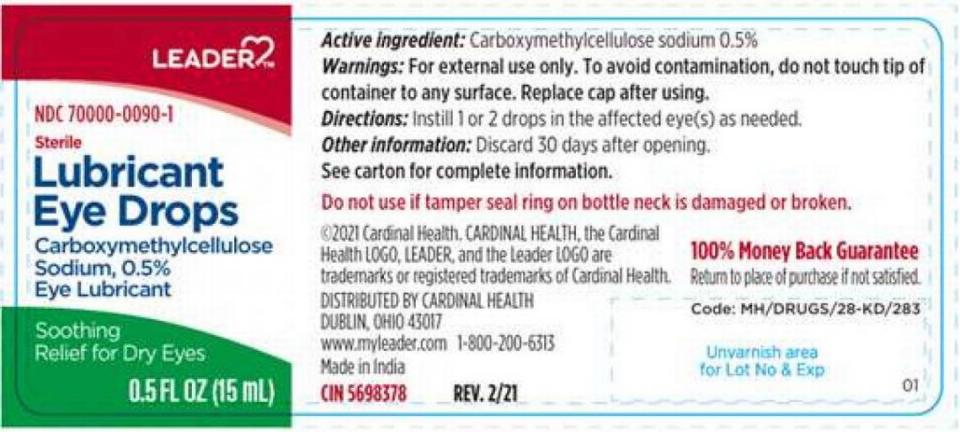
If you have these eye drops, return them to the store of purchase for a full refund. Your questions about this recall can be answered by emailing Cardinalhealth7720@sedgwick.com or calling Sedgwick at 855-215-4940, Monday through Friday, 8 a.m. to 5 p.m., Eastern time.
▪ The Harvard Group, working as Major Pharmaceutical and Rugby Laboratories, yanked all lots of 0.5-ounce (15 ml) bottles of Rugby Polyvinyl Alcohol, 1.4% Lubricating Eye Drops and Rugby Lubricating Tears Eye Drops (Dextran/Hypromellose, 0.1%/0.3%). Their nationwide distribution started June 1, 2021.

Return these eye drops to the store where you bought them for a full refund. For answers to questions about this recall, email harvarddrug8430@sedgwick.com or call Sedgwick, Monday through Friday, 8 a.m. to 5 p.m., Eastern time at 866-891-1981.

