Why did Labcorp and Quest fail to meet US COVID testing needs?
- Oops!Something went wrong.Please try again later.
On March 13, 2020, the day of President Donald Trump's Rose Garden press conference declaring COVID-19 a national emergency, his decision to rely on Labcorp and Quest Diagnostics for the bulk of the nation’s COVID-19 testing made sense. Only the "blood brothers" possessed the sample site networks, logistics operations, big laboratories and high-throughput machines we needed in a pandemic. And after decades of borrowing money to acquire their rivals, only Labcorp and Quest had the borrowing power and relationships with Wall Street banks to buy expensive, high-throughput test machines on credit.
“The consolidation in the industry got so that when COVID-19 hit, there were few other labs with the resources to invest in the costly equipment needed to do PCR testing,” said Mark Birenbaum, executive director of the National Independent Laboratory Association. “At the onset of the pandemic, they couldn’t take that risk.”
But even Labcorp and Quest have limits to how many test machines they can afford. As publicly traded companies that must return profits to shareholders, they can’t invest tens of millions of dollars in testing equipment built to last a decade for a pandemic that may end in months.
What was needed, experts told policymakers, was a unified national plan. Only the federal government can subsidize manufacturers to build more PCR machines, and laboratories to buy them.

That never happened.
“If it’s going to be gone by April [2020], they’re not going to invest in it. So they didn’t,” David Perlin, chief science officer at the Center for Discovery and Innovation, part of Hackensack Meridian Health, said about lab directors’ thinking early in the pandemic. “So it fell on folks like myself.”
Laboratories across the United States, including Labcorp and Quest, did scale up. But it could never be enough. By March 25, 2020, Quest Diagnostics reported a backlog of 160,000 COVID-19 tests, The New York Times reported. Smaller laboratories also were overwhelmed, so they sent their backlogs to Labcorp and Quest, exacerbating wait times.
“Quest and Labcorp became the labs of last resort. For everyone,” said Thomas Durant, an informatics researcher at Yale School of Medicine.
In June 2020, Quest said in a press release that its average turnaround time for a COVID-19 test was four to six days. In early July, Atlanta Mayor Keisha Lance Bottoms said she waited eight days for her family’s results. In mid-July, Labcorp and Quest announced that a spike of cases in the South and West was straining their systems. Additional surges in testing demand, long turnaround times, and spikes in COVID-19 deaths were reported in November 2020 and the spring and fall of 2021, plus the omicron surge, which caused long waits for results in the Northeast throughout the holidays and into January 2022.
“If you step back and look at our case fatalities, it’s just insane. It’s incomprehensible to me,” Perlin said. “We haven’t bought into a national strategy to protect each other. And until we do that, we’re going to be in the same place when the next thing comes.”
The delays rendered the results useless, both for protecting the health of individual patients and for limiting community spread of the disease. Criticism was harsh. Some observers accused Labcorp and Quest of profiteering.
“In Quest and Labcorp, you’ve got a duopoly,” said the economist Paul Romer. “Whenever you’ve got an incumbent with a big monopolized revenue stream, you know they’re going to resist any changes that might threaten that income.”
People with longer experience in the diagnostics industry say the situation was more nuanced.
In October 2021, Bob Roggeman of Quest Diagnostics said the company’s newest lab in New Jersey received regular deliveries of samples from Baltimore. Transfers like this make Quest and Labcorp two of the only entities capable of balancing the testing load between cities getting slammed by COVID-19 and cities where the outbreak is mild.
But absent massive federal subsidies, said Scott Gottlieb, former commissioner of the Food and Drug Administration, no private company can afford to spend hundreds of millions of dollars buying extra test machines, building bigger labs, or investing in higher throughput computers and logistics operations, all of which sit idle — generating zero revenue — until the excess capacity is needed in response to the next pandemic.
“It was a no-win situation” for Labcorp and Quest, said Mara Aspinall, a former testing company CEO and an adviser to the Rockefeller Foundation. “They couldn’t handle the demand. And they couldn’t say no.”
The omicron surge showed the limitations of a private system operating on its own, without government support. Throughout the holidays and into January 2022, CityMD walk-in clinics in New York City warned that due to laboratory backlogs, the average patient would have to wait five days for a COVID-19 test result. CityMD sends its samples to Quest Diagnostics for testing, said Michelle Maron, a Quest spokeswoman.

But it appeared that Quest’s vaunted national network did little to help the situation. Quest Air rarely flew to or from Teterboro Airport during the omicron surge, according to flight tracking websites. This suggests that any load balancing that occurred may have been accomplished using vans to transport backlogged samples to Quest’s smaller labs in the Northeast, which were experiencing omicron surges of their own, rather than to the company’s mega-labs in other regions where the surge hadn’t yet arrived, including ones in Lenexa, Kansas, and San Juan Capistrano, California.
Testing experts say this isn’t Quest’s fault. Rather, it’s a failure of public leadership, from the president down.
“I’m looking at people waiting in lines for three blocks waiting to be tested,” Perlin, of Hackensack Meridian Health, said in January 2022, as the latest surge died down. “Why is that? Everybody is overwhelmed. We don’t really have surge capacity right now.”
Conclusion: 'We knew what a disaster it was'
What to do? People who work in the scattered, dysfunctional landscape of diagnostic tests have ideas for how to fix it, but no confidence that politicians or the public are listening. One obvious suggestion most experts cite is nationalized health care. With a single federal agency paying all the bills, the standalone silos of hospitals and laboratories would lose the incentive to build walled-off kingdoms. They’d be forced to use a common computer system, with uniform rules about how data should be arranged and sent.
“The problem isn’t being able to run the test. The problem is we don’t have a national health care system,” said Kenneth Beckman, who operates the University of Minnesota Genomics Center. “There needed to be a central authority to take care of regulation, to establish best practices, and to cover your liability and insurance. Only a national health care system could have done that."

A smaller but related proposal is to assign every American a unique medical identification number. Both ideas were debated in 1993 in a health care reform package led by Hillary Clinton that came to be called “Hillarycare.” Both were roundly defeated. Neither seems politically feasible in the current climate.
“If everybody had a unique medical identification number, maybe we wouldn’t need some of those additional pieces of information with every lab result. We would have the key,” said Janet Hamilton, executive director of the Council of State and Territorial Epidemiologists. “But in this country, we don’t have the key.”
For decades, doctors and health experts have called on Congress to radically increase spending on public health. The CDC’s preparedness and crisis response programs received $858 million in 2020, a 50% reduction over the previous decade, according to the Trust for America’s Health, a nonprofit think tank. The Hospital Preparedness Program, the federal government’s only effort to help regional hospitals prepare for emergency, saw its budget drop from $515 million in 2004 to $275.5 million the year the pandemic struck. Funding from the CDC to help state and local public health agencies prepare for emergencies dropped by a third over the last decade, to $675 million in 2020.
When the Affordable Care Act was passed by Congress in 2010, it included $2 billion a year for prevention and public health programs. Later bills siphoned money away for other purposes, however, leaving public health programs underfunded by $7.5 billion, according to the trust.
Before the pandemic, groups including the Association of Public Health Laboratories spent more than a decade lobbying Congress for the money to create a national laboratory system capable of overseeing the testing response to a public health emergency. Congress consistently failed to respond, so the effort lapsed. Only later did the focus shift to modernizing information systems at public health agencies and laboratories that still depend on fax machines.
“We knew what a disaster it was,” Scott Becker, CEO of the Association of Public Health Laboratories, said of antiquated public health laboratory data systems. “And wow, did that prove to be the case.”
Whatever happens with funding, most testing experts agree that the United States still needs a unified plan to test for the next pandemic. The Trump administration famously ignored a pandemic response plan prepared by President Barack Obama’s administration. The Biden administration has continued Trump’s mistake, said experts including Emily Gurley and Jennifer Nuzzo of Johns Hopkins University.
“The problem was we never developed a national plan that was able to relieve [Quest and Labcorp] of the overwhelming burden they had,” said James Lawler, an infectious disease and public health expert at the University of Nebraska. “This is not a Trump administration issue. The current administration hasn’t done anything at all” to create a new national testing plan, Lawler said.
The federal government could address the testing shortage directly by creating a strategic reserve of test machines, said Gottlieb, the former FDA commissioner. Such a system might cost billions of dollars to create and run, said Gottlieb, who advocates for the idea.
In addition to his medical career, Gottlieb is a conservative political activist, having advised the presidential campaigns of Wisconsin Gov. Scott Walker and Donald Trump. After leaving Trump’s administration as FDA director, he returned to his previous job at the American Enterprise Institute, a right-wing think tank. That a leading member of the conservative establishment would suggest billions of dollars a year in new government spending is perhaps an indication that the situation is dire.
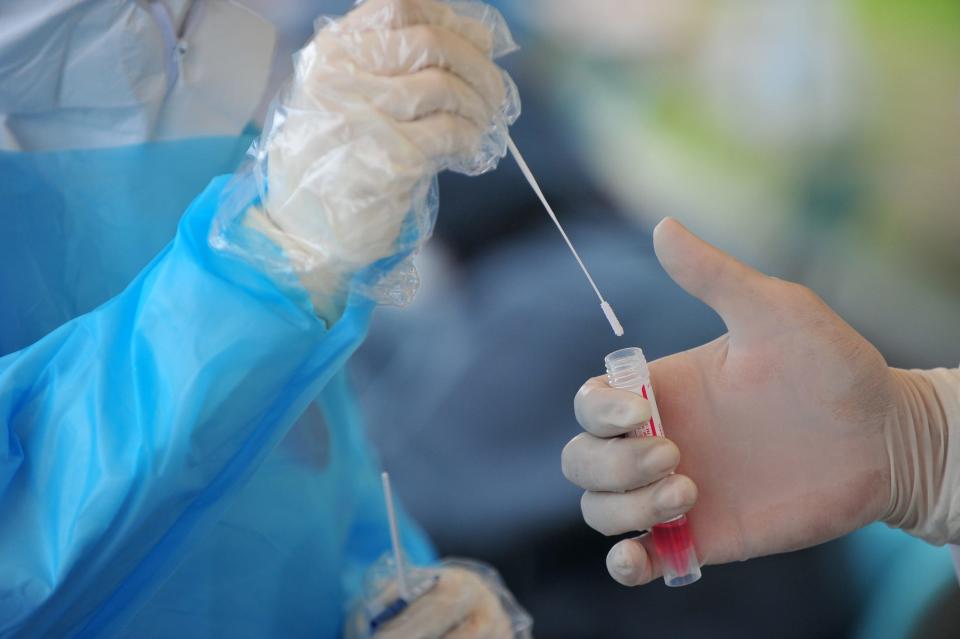
Yet for all his connections, Gottlieb sees no movement on his proposal.
“The policymakers are not focused on these structural issues yet,” he said.
Experts who propose smaller efforts meet the same silence. In April 2020, Lawler suggested that federal officials create a “Blue Apron”-style recipe for COVID-19 tests: specific enough to work, broad enough to work across all high-throughput testing platforms. The government never responded, he said.
At the beginning of the pandemic, there were indications Congress might be prepared to take the problem seriously. Public health data systems received $500 million from the 2020 CARES Act, plus about $600 million from subsequent appropriations, said Hamilton, of the epidemiologists council.
Experts viewed this as a nice down payment. Others call for even more funding:
The Trust for America’s Health recommends incremental increases, including $150 million in additional annual funding for the CDC’s public health preparedness response programs, and a boost of $50 million per year for the agency’s center for chronic disease prevention.
The Public Health Leadership Forum, a planning effort by a nonpartisan nonprofit called Resolve, has proposed that a functioning public health system would cost the United States an additional $4.5 billion a year.
The Rockefeller Foundation argues that simply bringing the nation’s testing infrastructure up to modern standards will cost $75 billion.
President Joe Biden’s original Build Back Better stimulus proposal included $36 billion for this work. His second, scaled-down version called for $16.2 billion. After the Build Back Better initiative failed in Congress, the only remaining proposal to improve public health data systems consisted of $150 million tucked into the Health and Human Services Department’s 2022 budget.
“This is what we do to public health. We fund it in a boom-and-bust cycle,” Hamilton said. “We can’t protect the health of millions of people if we’re not supported by rapid data systems.”
All of which means that in year three of the pandemic, Quest Diagnostics and Labcorp remain the nation’s primary backstop for diagnostic testing during a pandemic surge, just as they were before COVID-19. After retooling their sample sites to accommodate nasal samples, both companies got their silos of data, logistics and testing working again. Public health agencies, and major hospital chains including Northwell Health in New York and Yale New Haven Hospital in Connecticut, have scaled up their internal laboratories.
But they still have contracts with Labcorp and Quest for surge capacity. When hospitals and public agencies get overwhelmed, as they may again before this pandemic ends, they will send their excess samples into the duopoly’s networks and pray that, this time, Quest and Labcorp can handle the load.
“Everyone uses them for surge capacity,” said Durant, of Yale.
Through their trade group, the American Clinical Laboratory Association, Quest and Labcorp continue to lobby the Biden administration for various changes they say would improve the speed and scale of testing. Their proposals include an expansion of subsidies for patients to get tested, and making permanent a series of temporary regulatory changes implemented during the pandemic to allow laboratories greater flexibility in how they run existing tests, and bring new tests to market.
Experts say Quest and Labcorp can do more.
One simple step would be to force all labs to tell the federal government the number and types of test machines they own, Gottlieb said. This would empower federal and state officials to reduce testing bottlenecks during the next public health emergency by routing samples to labs with unused capacity.

“I know there were labs that, even in regions where there was overwhelming demand, they weren’t maxed out” during COVID surges, Gottlieb said. “There’s no central place to organize samples and make sure every lab is being used efficiently.”
One big difference since the beginning of the pandemic is that Quest Diagnostics and Labcorp have grown.
COVID-19 presented the greatest revenue bonanza in either company’s 25-year history. At Quest, annual revenues grew by $3 billion during the pandemic, to $10.79 billion in 2021. Labcorp’s market value doubled in a single year, to $24.9 billion in 2021.
Executives at both companies announced they’ll use the money to redouble their acquisition efforts, and buy still more of their competitors.
“Quest and Labcorp are using money from COVID to continue consolidation,” said Melissa Butterworth, president of Advanced Strategic Partners, which helps owners of small laboratories prepare their companies for sale to the duopoly. “They’ve really picked up their M&A activity, and they’re very aggressive.”
More revenue for Labcorp and Quest Diagnostics means more consolidation, bigger mega-labs, and fewer independent systems that can handle the load of monkeypox, the next COVID-19 variant outbreak, or the next pandemic.
“They’re private companies,” Beckman said. “It’s not their job to maintain tons of excess capacity for an event that everyone knows is coming.”
And just as before the pandemic, political leaders continue to ignore expert warnings that this is a mistake.
“What we do know is there will be other public health emergencies,” said Aspinall, the former testing company CEO, “and testing cannot be undervalued again.”
About the author
A columnist for NorthJersey.com and USA TODAY Network since 2016, Christopher Maag previously covered transportation, the environment, major economic development projects, and state government in New Jersey and New York.
Maag was named the Eugene C. Pulliam Fellow for Editorial Writing in August 2021. During his fellowship, Maag has been immersed in the medical testing industry, an obscure but burgeoning sector of the economy that has become the foundation of American healthcare.
Before joining The Record in 2013, Maag was a frequent contributor to The New York Times, Time, ABC News, Salon and Popular Mechanics, and a staff writer for monthly magazines, alternative news weeklies and web startups. He is a graduate of the Columbia University Graduate School of Journalism and Grinnell College.
Email: maag@northjersey.com
Twitter: @Chris_Maag
This article originally appeared on USA TODAY: Quest and Labcorp COVID testing efforts failed. Why?

