More than 500 deaths may be linked to recalled Philips sleep apnea machines, FDA says

The U.S. Food and Drug Administration (FDA) has issued an update to a recall affecting millions of Philips sleep apnea machines, saying that they may be linked to at least 561 reported deaths.
In a statement issued Wednesday, the agency said it has received more than 116,000 reports about the respiratory devices since April 2021, which have been found to break down and cause serious health hazards including choking, inhalation of foreign particles and increased risk of cancer.
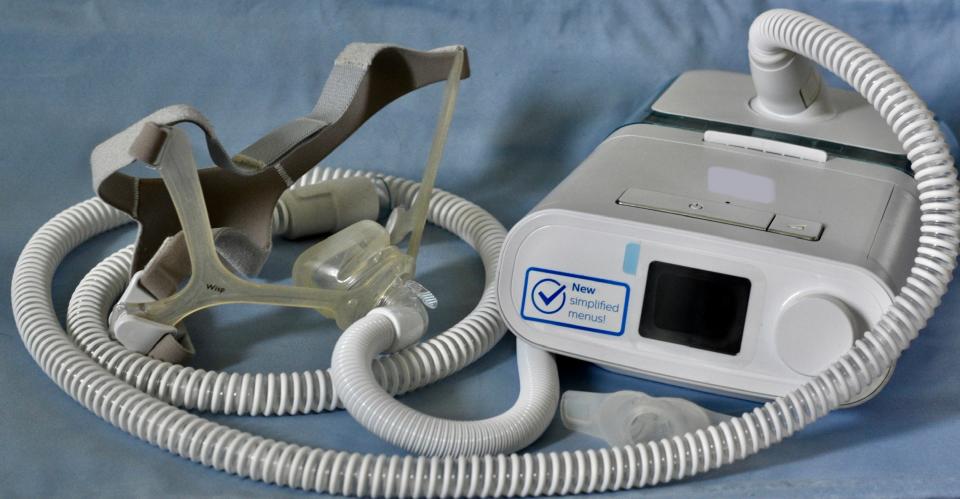
The devices, used for sleep apnea and similar sleep disorders, were made with polyester-based polyurethane (PE-PUR) foam, which has been found to break down over time and enter the airways of people using them. According to the FDA notice, the foam, which is used to reduce sound and vibration, degrades with use, causing "black pieces of foam, or certain chemicals that are not visible" to be "breathed in or swallowed by the person using the device."
Philips attempted to fix this issue after an initial recall of over 5 million devices in 2021.
Philips has since agreed with regulators to stop selling these and similar devices in the U.S., and a proposed class action settlement is underway. A spokesperson for Philips shared the following statement with USA TODAY.
“Based on the investigations to date, Philips Respironics has found no conclusive data linking these devices and the deaths reported in these medical device reports. Importantly, the submission of an MDR itself is not evidence that the device caused or contributed to the adverse outcome or event. Philips Respironics investigates all received complaints and allegations of malfunction, serious injury or death."
Here is what we know about the Philips sleep machine recalls.
Sleep apnea machines discontinued: Philips to halt sales of sleep apnea machines in US over ongoing safety concerns
Recalled Devices
ADHD medication recalled: FDA says bottles might have the wrong pills in them
Philips has agreed to stop selling sleep apnea machines in the U.S. and existing devices manufactured between 2009 and April 2021 are subject to recall, as they may pose a serious injury risk due to the breakdown of the PE-PUR foam used in them.
Devices included in the recall include:
A-Series BiPAP A30
A-Series BiPAP A40 (ventilator)
A-Series BiPAP Hybrid A30
A-Series BiPAP V30 Auto (ventilator)
C-Series ASV (ventilator)
C-Series S/T and AVAPS
DreamStation
DreamStation ASV
DreamStation Go
DreamStation ST, AVAPS
Dorma 400
Dorma 500
E30
Garbin Plus, Aeris, LifeVent (ventilator)
OmniLab Advanced+
REMstar SE Auto
SystemOne ASV4
SystemOne (Q-Series)
Trilogy 100 (ventilator)
Trilogy 200 (ventilator)
Certain Trilogy Evo ventilators with specific serial numbers.
Some products that were modified in an attempt to resolve the issue have been recalled as well. Specifically, certain reworked Philips Respironics Trilogy 100/200 Ventilators, as the FDA has asked for additional safety testing on the silicone foam material used to replace the PE-PUR foam.
These products include:
Trilogy Evo ventilator model numbers with certain serial numbers as listed in the recall database:
DS2110X11B
KR2110X15B (not distributed in the U.S.)
Repair kits for Trilogy Evo muffler assembly model and lot numbers as listed in the recall database:
Part number 1135257
Lot numbers between 210414 and 210524
Class action settlement and filing a claim
Philips settled with the FDA and U.S. Justice Department, agreeing to stop the sale of the affected products in the U.S. Philips has agreed to pay at least $445 million in compensation to users of the devices under a proposed class-action settlement for people who purchased a recalled Philips Respironics CPAP, BiPAP, or Ventilator sold in the U.S. between 2008 and 2021.
Eligible users can submit a claim to receive compensation via the settlement website. This does not prevent them from filing further action, meaning impacted people can still bring further action at a later date.
Those who qualify are entitled to:
A device payment award for each recalled device they purchased, leased or rented;
A device return award of $100 for each recalled device they purchased, leased, rented or were prescribed that they have already returned or that they return to Philips Respironics by August 9, 2024; and/or;
A device replacement award if they spent their own money to purchase a comparable CPAP, BiPAP, or ventilator between June 14, 2021, and September 7, 2023, to replace a recalled device.
People who believe they may be entitled to part of this settlement can check their eligibility and receive further instructions via the settlement administrator website.
The final date to submit a claim is August 9.
What are the symptoms of sleep apnea?
Sleep apnea is a common condition where breathing stops and restarts during sleep. The most common forms are obstructive sleep apnea and central sleep apnea, according to the National Heart, Lung and Blood Institute.
Obstructive sleep apnea occurs when the upper airways become blocked, reducing or completely halting airflow. Central sleep apnea happens when the brain fails to send signals to the regions of the body needed to breathe.
A common symptom of sleep apnea is loud snoring. Health experts also say people with sleep apnea tend to wake up multiple times during the night, sometimes gasping and choking. This is due to the brain responding to the lack of oxygen by alerting the body, forcing the person to wake up from sleep to restore normal breathing.
Patients with sleep apnea may experience abnormal breathing events, where airflow is reduced or stopped, from anywhere from five to 100 times in one hour, said Dr. Kin Yuen, sleep medicine specialist at UCSF Health and a spokesperson for the American Academy of Sleep Medicine.
“By the time we get to 100 events per hour, these are the people that have suffered a long time without seeking help and they’re more likely to have complete blockages,” she said.
Many people don’t know they have sleep apnea. The American Medical Association estimates that while about 30 million people may be living in the United States with sleep apnea, only 6 million are diagnosed.
Patients who don’t know if they snore or wake up in the middle of the night can identify sleep apnea through daytime symptoms, Yuen said, such as feeling fatigued during the day, mood changes, trouble concentrating and morning headaches.
How do you fix sleep apnea?
The gold standard of treatment for sleep apnea is using a continuous positive air machine, or CPAP, Yuen said.
The machine uses pressure to blow air in the upper airways, pushing away potential blockages, to maintain the respiratory cycle without interruption.
“It’s an important piece of our treatment for patients and so this whole Philips recall has created a tremendous amount of difficulty… in being able to treat our patients effectively,” she said.
Some people also need dental appliances or undergo surgery to correct structural problems in the jaw that could inhibit breathing.
What happens if sleep apnea goes untreated?
Sleep is the body’s way of repairing itself from the wear and tear of the day, Yuen said, and interrupting this natural process can wreak havoc on every part of the body.
Blood pressure, heart rate and blood sugar typically go down when a person is sleeping. But if they stay high due to lack of sleep, this could increase the risk of high blood pressure, cardiovascular issues and diabetes.
Insomnia due to sleep apnea can also damage a patient’s social-emotional health and increase the risk of mental health disorders, such as depression.
Can sleep apnea go away?
If a patient’s sleep apnea is due to structural problems, Yuen said, the condition won’t go away without corrective surgery.
However, there could be ways to improve sleep apnea if it’s caused by other factors.
People who rapidly gain weight are also more likely to develop sleep apnea as the excess tissues around the upper airways block airflow. Losing that weight could help reduce those blockages and improve sleep apnea.
Sleep apnea can also be caused by increased throat irritation or inflammation due to excessive drinking, smoking and vaping. Taking a break or abstaining from drinking and smoking could help decrease inflammation and improve airflow.
This article originally appeared on USA TODAY: Recalled Philips sleep apnea machines linked to 500 deaths: FDA

